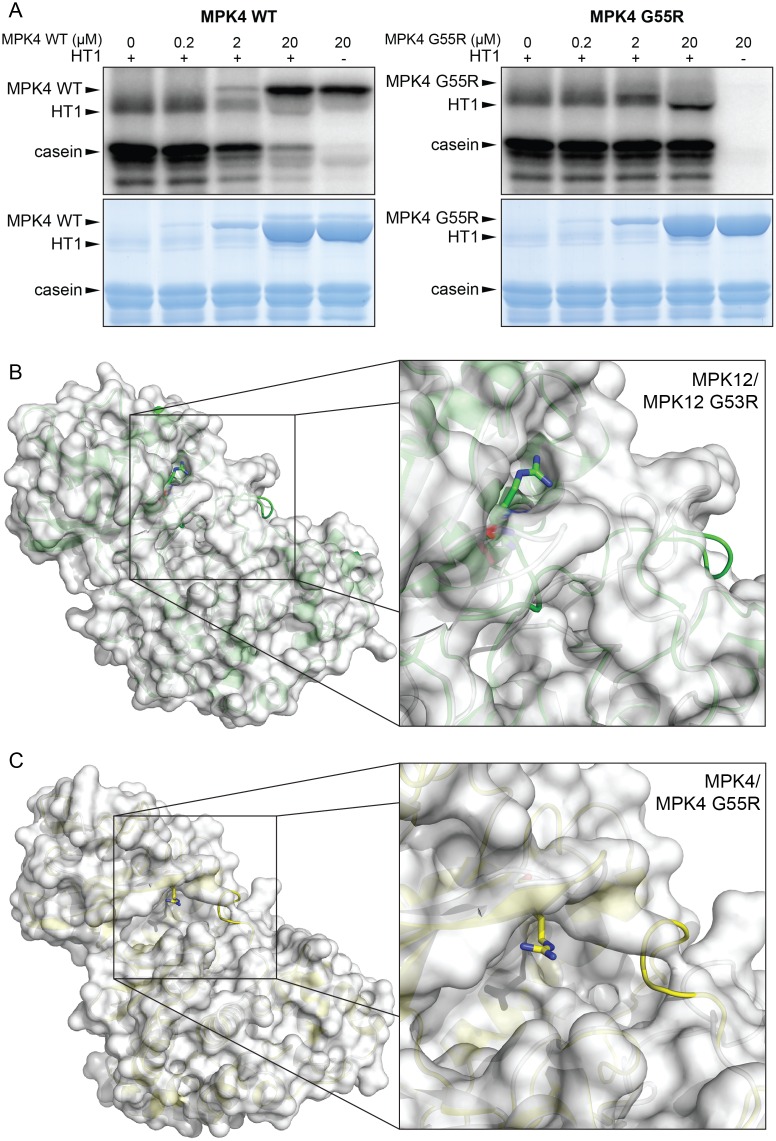
Fig 6. A conserved glycine is important for MPK4 and MPK12 function.
(A) Inhibition of HT1 kinase activity in vitro by MPK4 and MPK4 G55R. Upper panel: autoradiography of the SDS PAGE gel; lower panel: Coomassie-stained SDS PAGE. Reaction mixture was incubated for 30 min. (B) Whole protein (left) and close-up (right) view of the superposition of models for MPK12 wild-type (secondary structure and surface in white) and MPK12 G53R (secondary structure in green). There is a close structural similarity between the structures except where the arginine at position 53 protrudes from the mutant protein surface and changes the loop region for the mutant. (C) Whole protein (left) and close-up (right) view of the superposition of models for MPK4 wild-type (secondary structure and surface in white) and MPK4 G55R (secondary structure in yellow). Similar to MPK12 G53R, the arginine at position 55 in MPK4 protrudes from the mutant protein surface and changes the loop region.