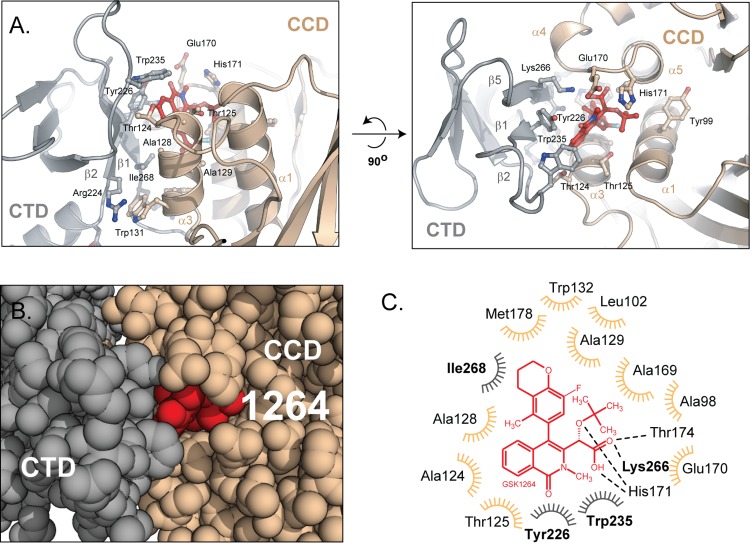
Fig 3. The GSK1264 binding interface.
(A) Orthogonal views of the GSK1264-induced polymer interface. GSK1264 is shown in red, packed between the CTD (grey) and the catalytic core domain dimer (tan). The positions of the side chains rendered are inferred from the high-resolution crystal structures used in the DEN refinement procedure. (B) Sphere rendering of the protein–ALLINI-protein interface. Colored as in A. (C) Schematic of IN-GSK1264 contacts. GSK1264 (red) is predominantly buried via van der Waals contacts with 13 residues from the CTD (grey) and catalytic core domain (tan). Thr174, Lys266, and His171 are predicted to hydrogen bond with the tert-butoxy and carboxylic acid moieties. This panel was generated using LIGPLOT [30]. See also S2 Fig, S3 Fig and S1 Table.