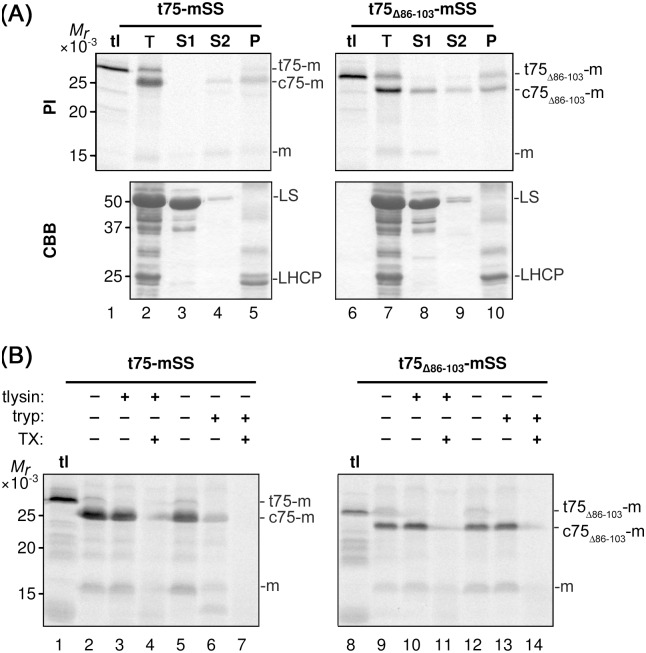
Fig 2. In vitro import of t75-mSS variants.
(A) Radiolabeled t75-mSS variants indicated above were incubated with isolated chloroplasts under the import condition. After 30-min of import, intact chloroplasts were reisolated and separated into two aliquots. The first aliquot was kept as total chloroplasts (T). The second aliquot was hypotonically lysed and fractionated by centrifugation to a supernatant (S1) and the pellet. The pellet was then resuspended with 0.1M Na2CO3 and fractionated by centrifugation to the second supernatant (S2) and the final pellet fraction (P). Samples equivalent to 3 μg chlorophyll were separated by SDS-PAGE, and radiolabeled proteins and total proteins in each sample were visualized by phosphorimaging (PI) and Coomassie Brilliant Blue staining (CBB), respectively. The experiments were done concurrently with those shown in Fig 3A and S1 Fig. tl contained the translation product corresponding to the one used for the import assay with 3 μg chlorophyll-equivalent chloroplasts. The precursors containing the entire t75 variants, the intermediates that carrying the c75 variants, and the mature forms lacking the entire t75 variant, respectively, are indicated at right; mSS is indicated with the letter m. For the CBB panel, large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase and light-harvesting chlorophyll a/b-binding protein are indicated as LS and LHCP, respectively. (B) After the import reaction as described in the legend to panel (A), intact chloroplasts were reisolated and separated into six aliquots. Three of them were resuspended in import buffer containing 1 mM CaCl2 with or without 1 μg thermolysin (tlysin) per μg chlorophyll equivalent chloroplasts and 1% Triton X-100 (TX) as indicated, incubated for 30 min on ice in the dark. Other three aliquots were resuspended in import buffer with or without 0.5 μg trypsin (tryp) per μg chlorophyll equivalent chloroplasts and 1% Triton X-100 (TX) as indicated, incubated for 60 min at room temperature in the dark. The activities of thermolysin and trypsin were quenched by 10 mM EDTA and 10 μg trypsin inhibitor per μg trypsin, respectively. Samples equivalent to 3 μg chlorophyll were separated by SDS-PAGE and radiolabeled proteins visualized by phosphorimaging. The experiments were done concurrently with those shown in Fig 3B and S2 Fig. For the labels at right, see the legend to panel (A).