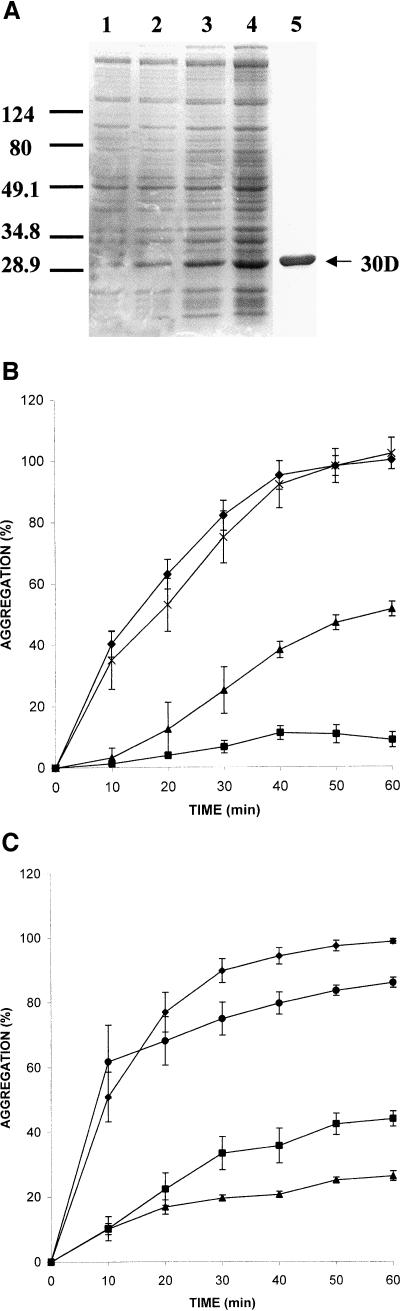
Fig 5.
Molecular chaperone activity of Xenopus Hsp30D. (A) Expression and purification of Hsp30D recombinant protein. Total bacterial protein from E coli BL21(DE3) cells containing the Hsp30D-pRSETB expression vector was collected before (lane 1) and after 5 hours, 14 hours, and 20 hours (lanes 2–4) of isopropyl-β-d-thiogalactopyranoside treatment. Recombinant protein was purified by means of nickel affinity column chromatography, as detailed in Fernando and Heikkila (2000). Protein was analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and visualized by Coomassie Brilliant Blue staining. Five micrograms of purified recombinant Hsp30D (30D) is shown in lane 5. Molecular mass markers in kDa are indicated on the left side of the figure. (B) Prevention of heat-induced citrate synthase (CS) aggregation by 30D. Aggregation assays were carried out using the protocols described previously (Fernando and Heikkila 2000). CS or luciferase (LUC) at 150 nM monomer concentrations was mixed with various molar amounts of 30D protein or incubated alone in a 50 mM N-2-hydroxythylpiperazine-N′-2-ethane-sulfonic acid–KOH (pH 7.5) buffer and heated at 42°C. Light scattering was determined at 10-minute intervals in a Beckman DU7 spectrophotometer at 320 nm. An increase in absorbance was indicative of protein aggregation. Data are representative of 4–6 trials and were calculated as a percentage of the maximum aggregation of CS or LUC after 60 minutes and expressed as the mean ± SE. CS was heat treated alone (♦, 0.1 μM) or in the presence of either 30D (▴, 0.1 μM; ▪, 0.5 μM) or IgG (x, 0.5 μM). (C) Inhibition of heat-induced aggregation of LUC by 30D. LUC was heat treated alone (♦, 0.1 μM) or in the presence of 30D (▪, 0.5 μM; ▴, 1.0 μM) or IgG (•, 0.5 μM)