Fig. 3.
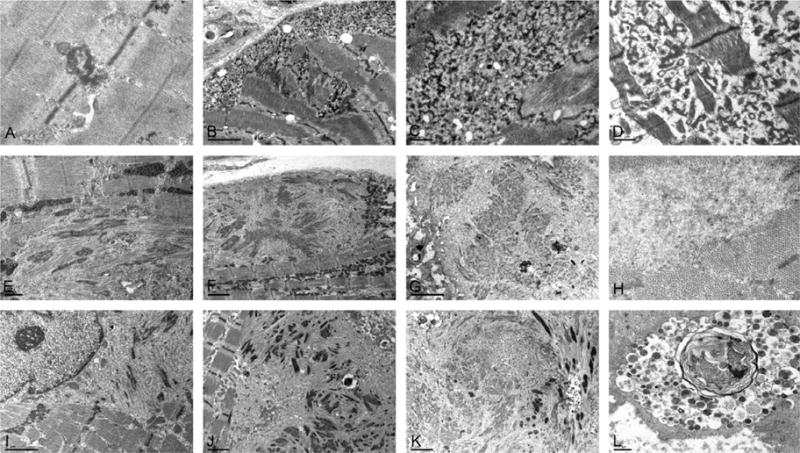
Ultrastructural findings in muscle biopsy samples from myofibrillar myopathy patients with DES, MYOT, or ZASP mutations. DES: early lesions consist of dappled electrondense structures emanating from the Z-discs (A). In more advanced lesions, a thick reticular network of electrondense granulofilamentous material forms in the subsarcolema and intermyofibrillar space (B, C); widening and streaming of Z-discs is also observed (B). Similar accumulation of granulofilamentous material is observed in cardiac muscle (D). MYOT: Dissolved myofibrils with disrupted Z-lines, first focally (E) and later spreading to larger areas and resulting in complete myofibrillar disorganization; multiple filamentous bundles of Z-disc origin and rod-like bodies (E, F), thin filaments and glycogen granules; groups of normal mitochondria surrounding the area of myofibrillar destruction (F); remants of filaments of various electron density accumulating into large inclusions (G); collections of 15–18 nm filaments are seen among normally looking myofibrils (H). ZASP: streaming and widening of the Z-line (I) is followed by sarcomeric disorganization and accumulation of filamentous bundles of Z-disc origin and filamentous debris (J); a spheroid inclusion composed of remants of filaments and rod-like bodies at the periphery (K); degraded material accumulates in autophagic vacuoles (L). A: p.Ile367Phe DES; B and C: p.Asp214_Glu245del DES; D: p.Arg406Pro DES E and F: p.Ser55Phe MYOT; G and H: p.Ser60Cys MYOT; I and J: p.Ala174Thr ZASP; L: p.Ala165Val ZASP. [Note: Figure shown in B is reproduced from Goldfarb L et al. (2010) Desminopathy. In Encyclopedia of Life Sciences. John Wiley & Sons Ltd: Chichester. DOI: 10.1002/9780470015902.a0006173.pub2, with permission from John Wiley & Sons Ltd.]
