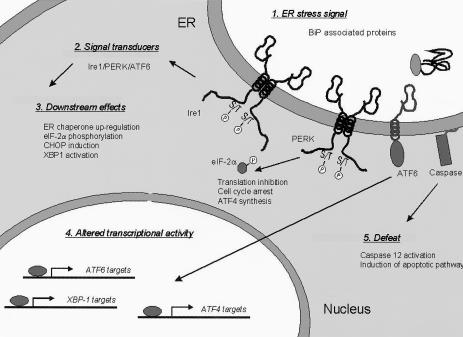
Fig. 1.
Aspects of the mammalian unfolded protein response (UPR) and the component of the pathway that regulates them. The signal for UPR activation is the accumulation of unfolded proteins that bind to the endoplasmic reticulum (ER) chaperone BiP. This signal is transduced by 2 transmembrane kinases (Ire1 and PERK) and an ER-tethered transcription factor (ATF6). PERK activation leads to eIF2-α phosphorylation, causing a general inhibition of protein translation, cell cycle arrest, and ATF4 synthesis. Other downstream effects of UPR activation include the transcriptional up-regulation of ER chaperones, CHOP induction, and XBP-1 messenger ribonucleic acid cleavage, resulting in an altered form of the XBP-1 transcription factor. UPR activation is accompanied by alterations in the transcription of a number of genes, most notably those regulated by ATF6, ATF4, and XBP-1. If the stress conditions persist, apoptotic signaling pathways are activated, in part, through the cleavage of procaspase 12