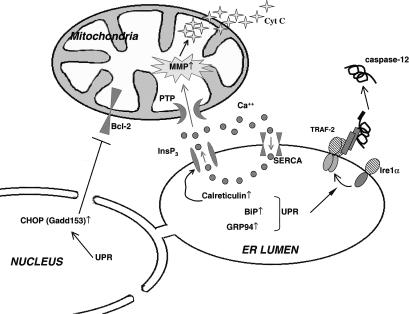
Fig. 2.
Illustration of unfolded protein response (UPR)-dependent apoptotic pathways. Upon endoplasmic reticulum (ER) stress, oligomerization of Ire1α induces the binding of TRAF-2 to its cytoplasmic domain, which in turn promotes procaspase-12 clustering, cleavage, and activation. UPR activation also up-regulates ER chaperones like BiP and GRP94, which can bind calcium and prevent further calcium mobilization during ER stress. Conversely, calreticulin, another UPR-induced ER chaperone, regulates the function of the ER inositol-1,4,5-trisphosphate (InsP3) calcium channel to increase calcium mobilization to the cytosol. The released calcium activates the mitochondria permeability transition pore (PTP) resulting in mitochondrial membrane permeabilization. This causes cytochrome c release from the mitochondria, leading to activation of apoptosomes. Finally, the UPR induces CHOP, which decreases the expression of the antiapoptotic protein Bcl-2, further contributing to cell death