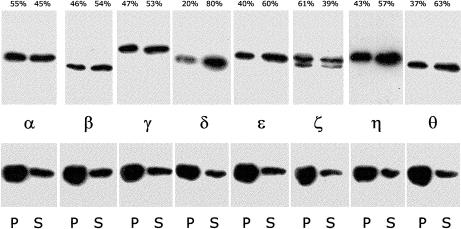
Fig 3.
Immunoblot quantitation of selective type-II chaperonin containing T-complex polypeptide 1 (CCT) subunit binding to F-actin in a polymerization experiment. After incubation under polymerizing conditions for 2 hours at room temperature, a sample containing 4.5 μM actin (method 2—see text) and 0.05 μM CCT (a single column fraction—see text) was centrifuged at 96 600 × g for 30 minutes and corresponding volumes of pellet (P) and supernatant (S) prepared for sodium dodecyl sulfate–polyacrylamide gel electrophoresis and Western blot. Upper panels are strips that were probed separately with CCT subunit-specific antibodies, as indicated. Lower panels are the same blot strips reprobed with anti-actin (monoclonal antibody C4 from ICN Pharmaceuticals Inc. Costa Mesa, CA, USA) yielding 73% of the immunoreactivity in the pellet vs 27% in the supernatant. These latter values were consistent across lanes and intensities indistinguishable, suggesting uniformity of transfer to nitrocellulose during electroblotting