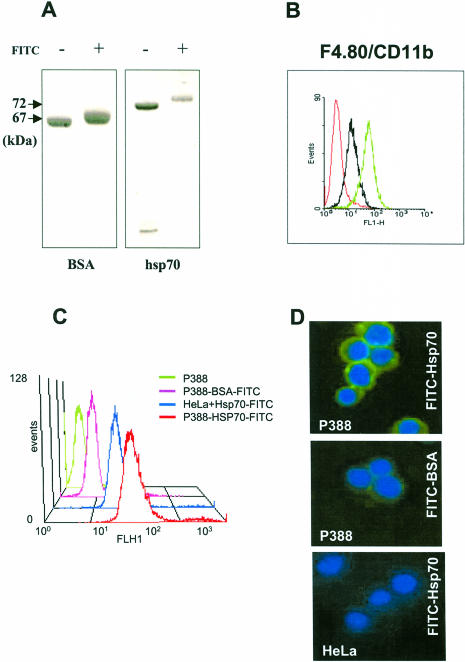
Fig 1.
P388 macrophage-like cells specifically bind heat shock protein 70 (Hsp70). Human recombinant Hsp70 was expressed and purified as described elsewhere (Zimmer et al 2001). Five hundred to seven hundred micrograms of protein was incubated with fluorescein isothiocyanate (FITC) (Molecular Probes Inc. Eugene, OR, USA) in phosphate-buffered saline at a molar ratio of 1:10 for 2 hours at room temperature. Nonincorporated FITC was removed with a Micro Bio-spin column (BioRad Laboratories GmBH, Vienna, Austria; cutoff value of 40 kDa). The number of conjugated FITC molecules was estimated by measuring the optical density of the samples at 280 nm and 480 nm, and the labeling efficiency was ensured by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (A). Labeled proteins were incubated at 4°C for 1 hour with mouse P388 cells that were previously analyzed by FACS (B) for CD11b surface positivity (green line) and F4/80 surface staining as a positive control (black line). Red line indicates unstained control cells. Unbound proteins were removed by extensive washing, and surface binding was ensured by FACS analysis (C) and fluorescence microscopy (Zeiss Axiophot) (magnification, 400×) (D). Nuclei were stained with 4′,6-diamidino-2-phenylindole dihydrochloride