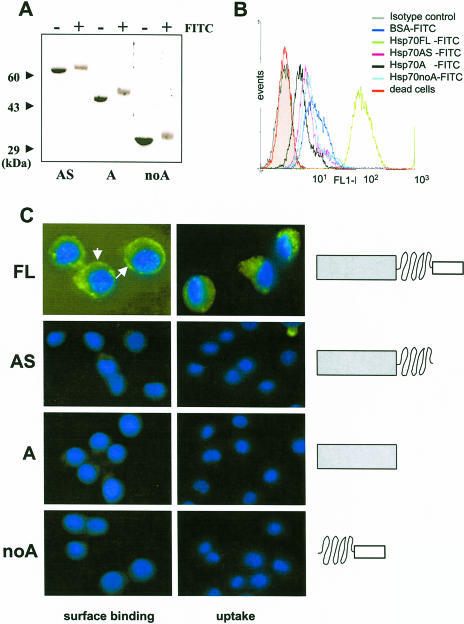
Fig 3.
Surface binding and internalization of full-length (FL) heat shock protein 70 (Hsp70) and its deletion mutants by P388 cells. Deletion mutants of Hsp70 were generated as described elsewhere (Zimmer et al 2001) and were labeled with fluorescein isothiocyanate (FITC) as described for the FL protein (see legend to Fig 1). Labeling efficiency was ensured by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (A). Cells were incubated in the presence FITC-labeled proteins as described in materials and methods section, washed extensively, and analyzed by FACS (B) and fluorescence microscopy (C) as described in Figure 1. Nuclei were stained with 4′,6-diamidino-2-phenylindole dihydrochloride. White arrows indicate the spherical surface localization of labeled Hsp70 by P388 cells. Magnification was 630-fold for the FL protein and 400-fold for the other mutants. Schematic illustration of the domain composition of wild-type and mutant Hsp70 proteins is presented in the right of panel C