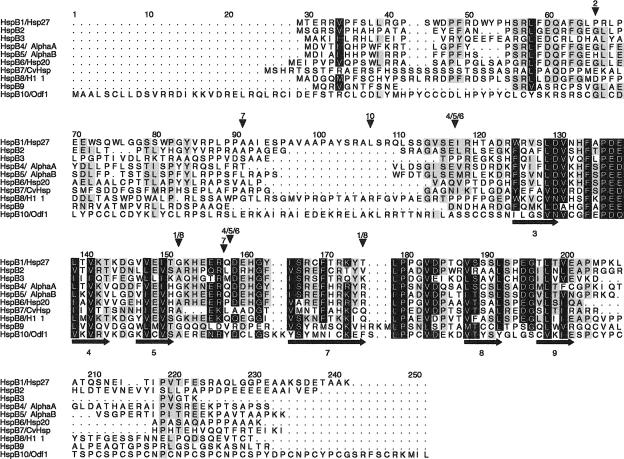
Fig 1.
Alignment of the 10 human small heat shock proteins (sHsps). Intron positions and predicted secondary structure elements are indicated by arrowheads above and arrows (for ß strands) below the alignment, respectively. The α-crystallin domain is approximately located between positions 118 and 203. Sequences correspond to the protein accession numbers in Table 1 (but see footnote i for HspB6). The alignment is made with ClustalW and manually edited in GeneDoc. Residues in black are conserved in 8 or more sHsps and those in gray in 5–7. The secondary structure was predicted from the 10 aligned sequences with PHD (Rost and Sander 1994); predicted ß strands are numbered according to the approximate location of the corresponding ß strands in the crystal structures of M jannaschii Hsp16.5 (Kim et al 1998) and wheat Hsp16.9 (van Montfort et al 2001). Numbers above the arrowheads correspond to the HspB1–10 numbering: thus, in the HspB1 and HspB8 genes, 2 introns are present at the same positions, 152 (phase 1) and 175 (phase 2); HspB2 has a single phase 1 intron, at position 66; HspB3 and HspB9 are intronless; HspB4, HspB5, and HspB6 have 2 phase 0 introns at identical positions, between 117 and 118 and between 158 and 159; HspB7 has 2 introns, at positions 91 (phase 1) and between 157 and 158 (phase 0); HspB10 has 1 phase 2 intron, at position 107