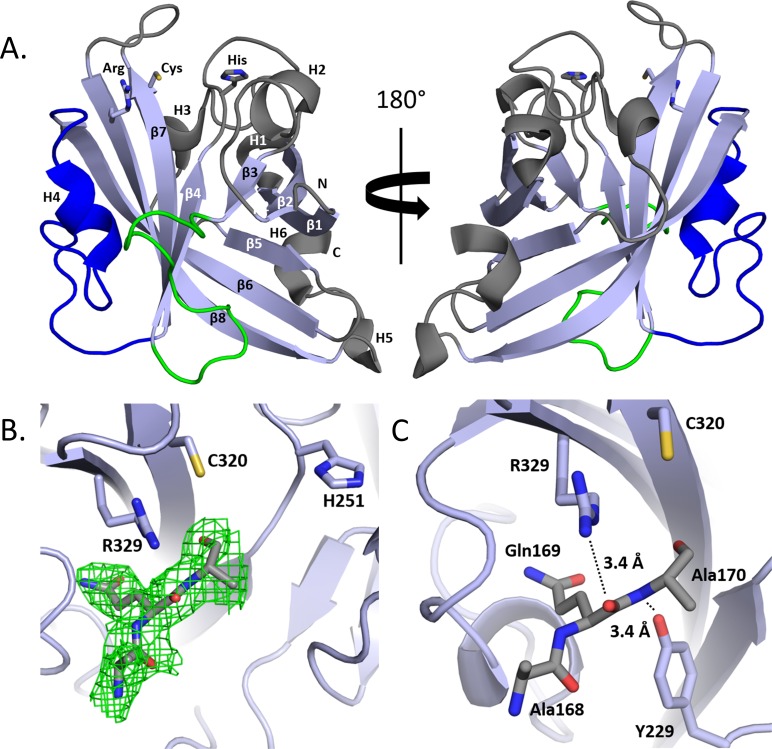
Fig 2. Crystal structure of SrtE1 from S. coelicolor.
A) Ribbon diagram of the SrtE1catalytic domain with the conserved Arg-Cys-His triad shown in sticks. Beta sheets (β) and helices (H), as well as N- and C-termini, are labeled accordingly. The sortase β-barrel core (light blue), structurally unique β3/β4 (green) and β6/β7 loops (blue), and accessory loops and helices (gray) are colored. B) An Fo−Fc omit map of the active site contoured at +3 σ (green mesh). The map was generated by omitting the AQA tripeptide from the final model and performing additional refinement. The omit density accommodates an AQA tripeptide adjacent to Arg329 and Cys320 within the active site. C) Interactions between SrtE1 active site residues and AQA tripeptide. Potential hydrogen bond interactions (black dashed line) between R329, Y229, and AQA tripeptide (sticks) are shown.