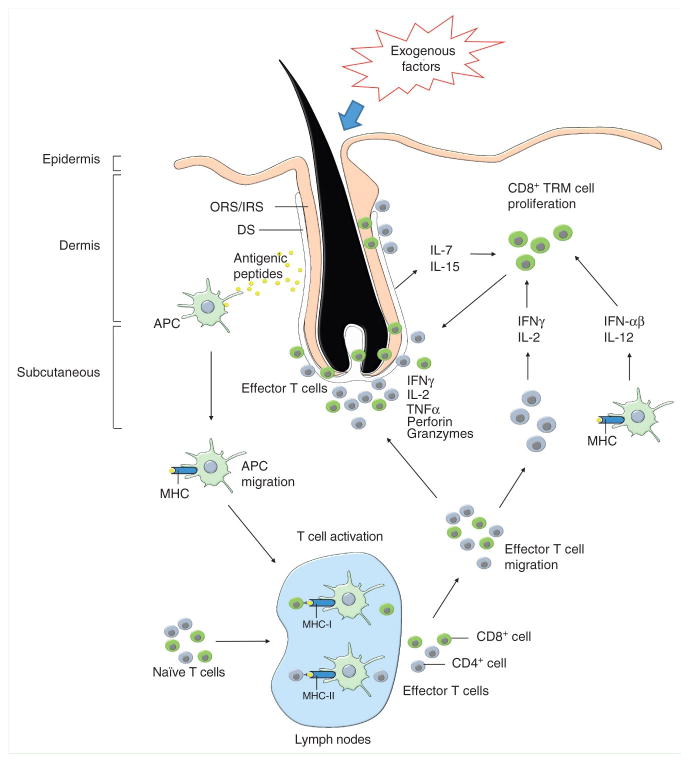
Figure 1. Effector CD8+ T cells plays a critical role in initiating alopecia areata.
Changes in normal hair follicle parameters in response to exogenous factors, such as infectious agents and/or trauma, in conjunction with endogenous factors, such as elevated stress hormones and/or genetic susceptibility, elicits activation of skin resident antigen presenting cells (APCs). APCs process hair follicle specific antigen epitopes in the presence of proinflammatory signals. Activated APCs migrate to skin draining lymph nodes to mature and present the pathogenic peptides to naïve T cells. Upon recognition of peptide-major histocompatibility complexes (MHC), in association with proinflamatory costimulatory signals on the surface of APCs, naïve T-cells proliferate and differentiate into effector T cells. Then, the effector T cells migrate to the skin and accumulate around anagen stage hair follicles. Primarily CD8+ T cells penetrate to intrafollicular locations to initiate the autoimmune attack in response to autoantigens in anagen stage hair follicles, while CD4+ cells remain in perifollicular locations. Hair follicle disruption may be mediated via pro-apoptotic signals such as perforin and granzymes, and/or hair follicle regression may be induced via inflammatory cytokines. In addition to the inflammatory lymphocyte infiltrate, inflamed hair follicle keratinocytes in dystrophic hair follicles release cytokines, including IL-15 and IL-7, and may induce tissue resident memory T cell proliferation. The activated memory T cells recruit new autoantigen-specific memory T cells and bystander cells from the circulation and further support direct disruption of hair follicle cells.