Table 2.
Distortion/Interaction Analysis for the Cycloaddition of Diazoacetamide 1 (R = Me) with Alkynes 3a and 3b and with ONDs 4a and 4ba
|
Δ
G
‡
dist
|
charge (e−) on the dipole (1) in the TSb |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
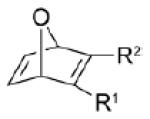
| dipolarophile | R1 | R2 | Δ E ‡ | Δ G ‡ | dipolarophile | dipole (1) | total | Δ G ‡ int | gas-phase | MeOH | Δ | |
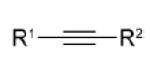
|
3a | CO2Me | CF3 | 9.6 | 21.5 | 10.4 | 14.8 | 25.2 | −3.6 | 0.25 | 0.28 | 0.3 |
| 3b | CO2Me | CO2Me | 9.8 | 22.8 | 12.7 | 13.1 | 25.8 | −3.0 | 0.26 | 0.28 | 0.2 | |
|
4a | CO2Me | CF3 | 8.2 | 22.1 | 8.1 | 16.2 | 24.3 | −2.2 | 0.24 | 0.25 | 0.1 |
| 4b | CO2Me | CO2Me | 7.8 | 22.5 | 6.4 | 17.7 | 24.1 | −1.6 | 0.22 | 0.23 | 0.1 | |
Calculations were performed at the M06-2X/6-31+G(2d,p) level of theory. Energies (kcal/mol) include solvation corrections (MeOH) on gas-phase geometries with the IEFPCM model (radii = UFF);
Calculated with natural bond orbital (NBO) analysis.18
