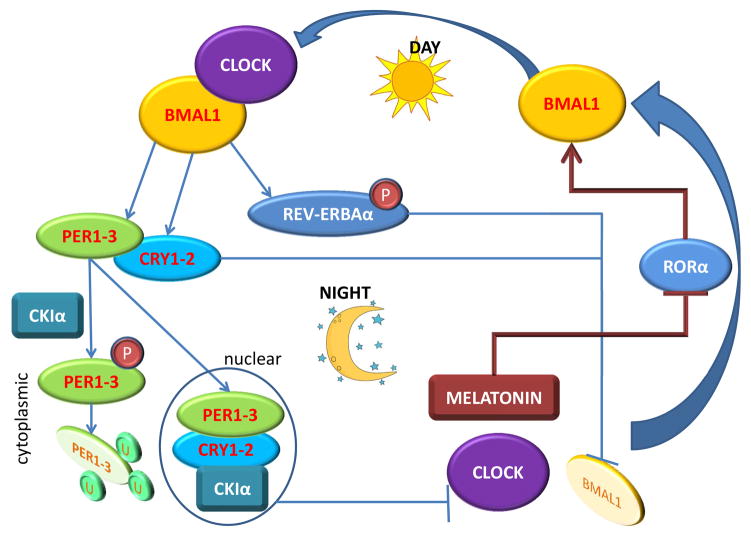
Figure 3. 24-hour cycle of the circadian clock.
The expression of Clock itself does not significantly vary during the 24-hour cycle, but it is only one of few genes in this group that does not. Major regulation of the circadian clock is mediated by the orphan nuclear receptors RORα and REV-ERBAα which determine the expression of Bmal1 (also known as ARNTL), the key binding partner of Clock. During the day, the Clock-Bmal1 dimer transcribes genes that result in the synthesis of Per and Cry, as well as REV-ERBAα. As the day progresses, Per and Cry dimerizes and forms a stable complex that translocates to the nucleus. Any free Per remaining in the cytoplasm is phosphorylated by CKIε, which causes it to be ubiquitinated and degraded. As night falls, the Per/Cry oligomer complex in the nucleus disrupts the formation of the Clock-Bmal1 heterodimer, which then stops producing Per and Cry. In addition, REV-ERBAα is phosphorylated by GSK3β which stabilizes the protein, and allow it to transcriptionally repress the expression of Bmal1. Bmal1 levels are also suppressed when melatonin levels increase in the circulation, which inhibits the expression of RORα that positively regulates Bmal1 expression. Decrease in Bmal1 further disrupts the Clock-Bmal1 complex and prevents the transcription of target genes, including Per and Cry. As REV-ERBAα levels in the cell, and melatonin levels in the circulation, decrease early in the morning, Bmal1 synthesis is resumed. Further, Per and Cry levels in the nucleus also fall off due to decreased synthesis, and Clock-Bmal1 complexes resume, which then allows the circadian clock to move forward. P-denotes protein phosphorylation, U- denotes protein ubiquitination.