Figure 3. Nitroxidative mechanisms of neuroexcitability after tissue injury.
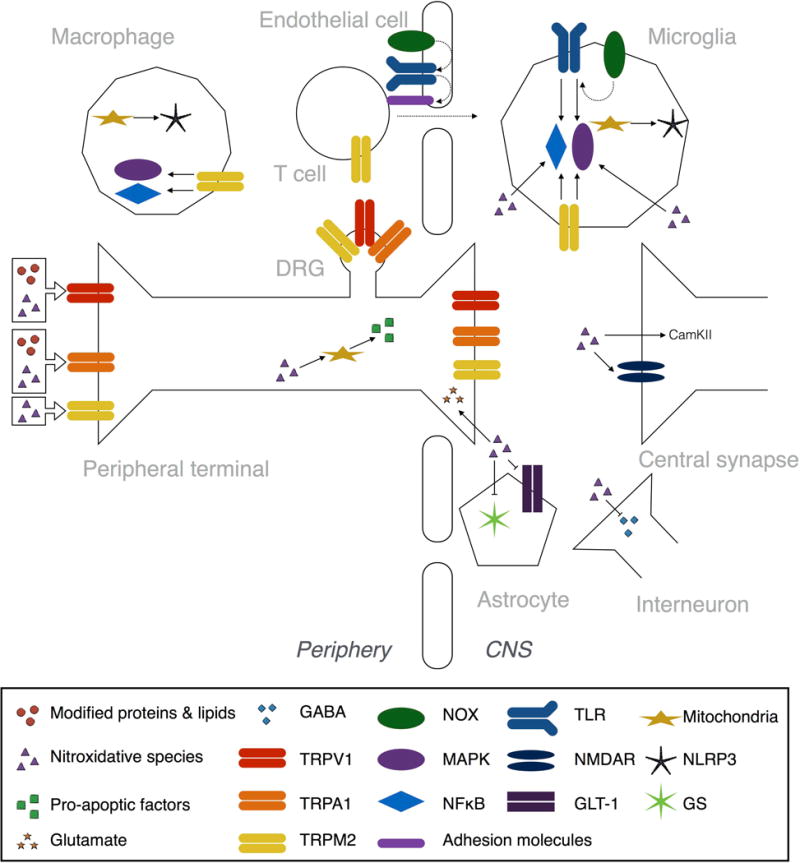
Reactive nitroxidative species, such as hydrogen peroxide and peroxynitrite, and modified proteins and lipids, like carbonylated proteins, peroxidated and nitrated lipids, and reactive aldehydes, all contribute to peripheral and central sensitization after tissue injury. These processes drive pathological pain. Several of the Transient Receptor Potential (TRP) family of nonselective cation channels are activated by nitroxidative species and modified proteins and lipids (see Nitroxidative species activate TRP channels). TRPA1 is expressed by peptidergic C-fibers, and is activated by modified proteins and lipids. TRPM2, which is expressed by neurons, monocytes/macrophages, microglia, and T cells, is directly activated by nitroxidative species. TRPM2 also activates intracellular signaling pathways, including mitogen activated protein kinase (MAPK) and nuclear translocation of nuclear factor κ-light-chain-enhancer of activated B cells (NFκB) pathways. TRPV1 is found on C-fibers and is directly activated by some modified proteins and lipids, as well as being a target of oxidation and nitration events by nitroxidative species that increase responsiveness of the channel. Reactive nitroxidative species can directly modulate neuroexcitibility in central synapses by promoting glutamate release from primary afferent terminals, by activating calcium calmodulin-dependent protein kinase II (CamKII) in glutamatergic spinal neurons, and by inhibiting GABAergic interneurons (see Nitroxidative species as neuromodulators in pain pathways). Nitroxidative species also disrupt glutamate homeostasis by nitration and phosphorylation of NMDA receptor (NMDAR) subunits, as well as inhibiting glutamine synthetase (GS) and the glutamate transporter GLT-1. Mitochondrial DNA is a target of oxidation and nitration, while some nitroxidative species can form adducts with many mitochondrial proteins, which together impairs the structural integrity and function of mitochondria (see Nitroxidative species induce mitochondrial dysfunction). Nitroxidative species can also trigger release of pro-apoptotic factors from mitochondria by disrupting organelle dynamics. Nitroxidative species induce production of proinflammatory mediators, and can activate NFκB and MAPK intracellular signaling pathways (see Nitroxidative species induce neuroinflammatory signaling). Toll like receptors (TLRs) bind a variety of endogenous danger signals, including those released from nitroxidative-damaged mitochondria, to activate NFκB and MAPKs. NOX-derived ROS are second messengers for NFκB- and p38 MAPK-dependent TLR signaling, and TLR expression. The TLR2-NOX1 interaction also upregulates adhesion molecules via CCL3, which facilitates transendothelial cell migration into the CNS. Mitochondria-derived ROS also activate NLRP3 inflammasomes, which are protein complexes responsible for the proteolytic activation of IL-1β.
