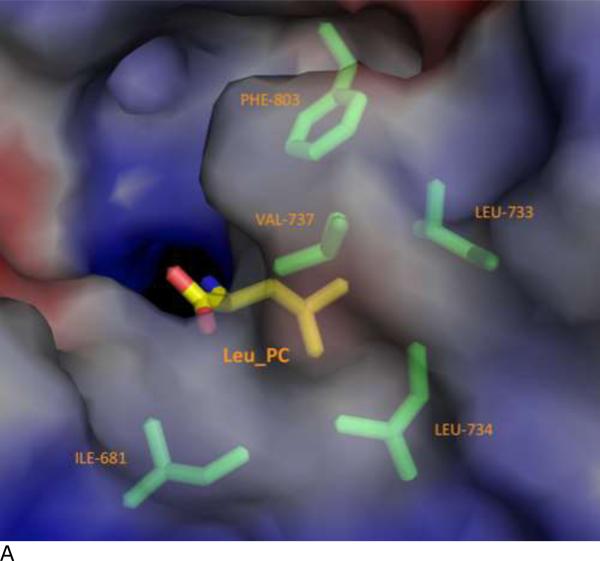
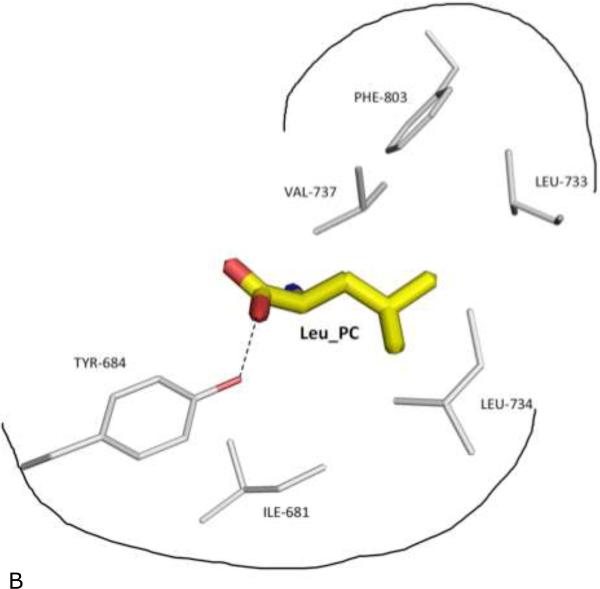
Figure 4. Specificity pocket on ERAP1 regulatory domain for binding peptide's PC anchor.
(A) The C terminus binding cleft of ERAP1 is shown as solvent accessible surfaces colored by electrostatic potentials: from red to blue for negatively charged to positively charged areas. The peptide's Leu anchor at PC position (LEU_PC) is shown as a thick stick model colored by atom types: yellow for carbons, blue for nitrogen, and red for oxygens. ERAP1 residues surrounding the LEU_PC side chain are shown as thin stick models colored by atom types: green for carbons, blue for nitrogens, and red for oxygens.
(B) Schematic outline of the specificity pocket, with surrounding side chains of ERAP1 residues shown and labeled. Also shown is Tyr-684 of ERAP1 that makes direct contact (dotted hydrogen bond) with the PC carboxylate end.