Figure 1.
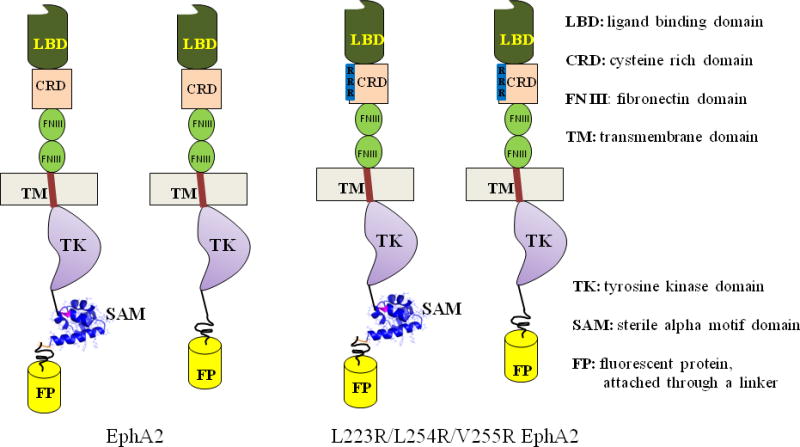
The four EphA2 constructs investigated in this work to elucidate the role of the EphA2 SAM domain in receptor dimerization. For these measurements we used both the wild-type and the EphA2 L223R/L254R/V255R mutant, which has lower dimerization ability due to extracellular mutations in a receptor-receptor interface. Both EphA2 wild-type and mutant were analyzed in their full-length version and in a version lacking the SAM domain.
