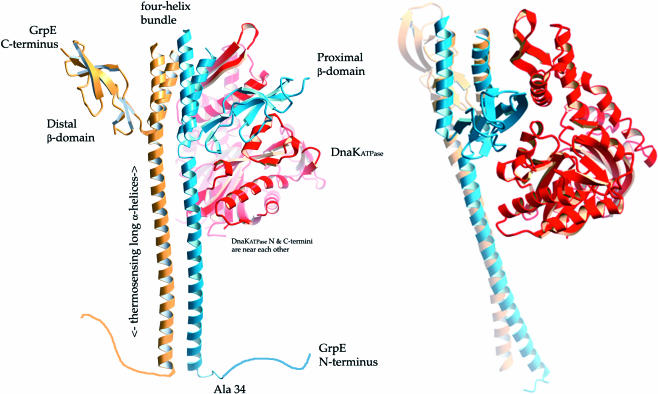
Fig 1.
Ribbon drawings of dimeric GrpE bound to the adenosine triphosphatase (ATPase) domain of DnaK (PDB: 1DKG). GrpE monomers are colored blue and gold, and the ATPase domain is colored red. In the cocrystal structure, the connecting loops at the top of the 4-helix bundle were too disordered to be modeled. The unstructured N-terminal 33 amino acids of GrpE are drawn as a random coil. These residues have been implicated in interactions with the DnaK substrate-binding domain and were removed by proteolysis before crystallization. GrpE amino acids 34–39 were either unstructured (blue monomer) or too disordered to be modeled (gold monomer) in the cocrystal structure. The bottom of the long α-helices corresponds to residue 40 of GrpE. It can be seen that the compact β-sheet domain on the GrpE monomer proximal to DnaK sits at the top of the DnaK nucleotide-binding cleft, wedging it open. The figure on right is rotated by ∼70° about the vertical axis with respect to the figure on the left. These figures are rendered with “fog” to provide depth cueing. This figure was made with Molscript (Kraulis et al 1991), converted to a POVRAY-style format (D. Jeruzlami, unpublished software), and rendered with POVRAY (www.povray.org).