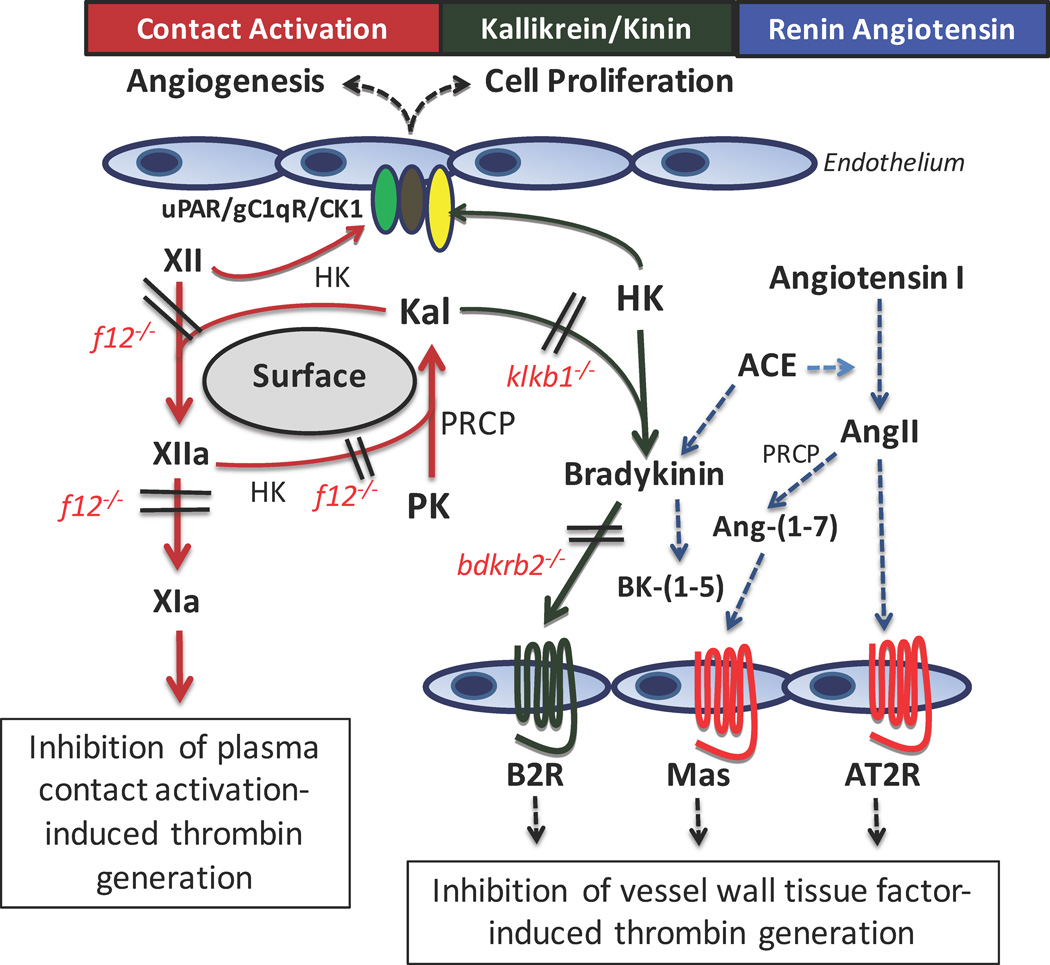
Figure 1.
Antithrombotic potential of the contact activation and kallikrein/kinin systems. The contact activation system (CAS) consists of factor XII (XII) auto-activating on surfaces (biologic or artificial) to form factor XIIa (XIIa) that then reciprocally activates prekallikrein (PK) to form plasma kallikrein (KAL) with amplification of activation of each zymogen. The system is accelerated by the presence of the cofactor to XII and PK activation, high molecular weight kininogen (HK). Another serine protease, prolycarboxypeptidase, (PRCP) also activates PK to KAL independent of factor XIIa. Formed factor XIIa also activates factor XI (XIa) leading to contact activation-induced thrombin generation. Deletion of FXII (f12−/− mice) or inhibition of FXIIa blocks contact activation-induced thrombin generation. This pathway is most operative with device-related thrombosis. FXII also has a multiprotein receptor system on endothelium that consists of uPAR, gC1qR, and cytokeratin 1 (CK1) that when stimulated induces cell proliferation and angiogenesis. HK binds to the same receptor system to block these functions of FXII.
The kallikrein/kinin system (KKS) consists of formed plasma kallikrein (Kal) by any mechanism (XIIa, PRCP) cleaving HK to liberate bradykinin (BK). BK binds to its constitutively expressed receptor, the bradykinin B2 receptor (B2R). In PK deficient (klkb1−/−) mice, reduced BK formation and B2R expression is associated with increased amounts of the G-protein coupled receptor Mas. Similarly, but to a greater extent, in B2R deleted (bdkrb2−/−) mice, the absent receptor is associated with increased expression of the G-protein coupled receptors Mas and angiotensin receptor 2 (AT2R). Further, it has elevated BK that is metabolized by angiotensin converting enzyme (ACE) of the renin angiotensin system (RAS) to produce bradykinin-(1–5) (RPPGF). The increased ACE activity also produces more angiotensin II (AngII) from angiotensin I. The excess AngII binds the AT2R or is metabolized by PRCP or ACE2 (not shown) to angiotensin-(1–7) [Ang-(1–7)] to stimulate the Mas receptor. Ang-(1–7) is the natural ligand of Mas. The combined AngII and Ang-(1–7) agonism on the AT2R and Mas, respectively, results in a 2–3 fold increase in prostacylin. In klkb1−/− mice, constitutive levels of Ang-(1–7) is sufficient to stimulate the over-expressed Mas receptor to increase prostacyclin production 1.5–2.0-fold. The increased prostacyclin in both animal models then leads to a cascade of downstream signals that first results in reduction of vessel wall tissue factor in addition to reduced contact activation.