Abstract
Background
Overexpression of matrix-metalloproteinases (MMPs) has been shown to lead to tissue damage in equine recurrent airway obstruction (RAO), as a misbalance with their natural inhibitors, the tissue inhibitors of metalloproteinases (TIMPs), occurs. This favors irreversible pulmonary fibrosis formation. Increased levels of MMPs, TIMPs or altered ratios between them can be used as biomarkers of respiratory disease. We hypothesized that levels of MMPs, TIMPs and their ratios correlate with improvement in clinical findings and bronchoalveolar lavage fluid (BALF) cytology after 10 days of inhalative glucocorticoid therapy and environmental dust reduction (EDR) and may be used to monitor treatment success.
Ten horses with a history of RAO participated in a prospective clinical study. Clinical and cytological scoring was performed before and after inhalative therapy using budesonide (1500 μg BID over 10 days) and EDR (bedding of wood shavings and wet hay as roughage). Gelatin zymography was performed for qualitative and semi-quantitative evaluation of MMP-2 and MMP-9 in BALF supernatant, while fluorimetry was used to evaluate MMP-8 activity. Additionally, specific equine ELISA assays were used for quantitative assessment of MMP-2, MMP-9, TIMP-1 and TIMP-2.
Results
A significant reduction in the total and several single parameters of the clinical score were found after 10 days of inhalative therapy and EDR. The concentrations of MMP-2, MMP-9, TIMP-1 and TIMP-2 (ELISA) as well as their activities (MMP-2 and MMP-9 zymography and MMP-8 fluorimetry) were significantly decreased after therapy. Significant improvements in MMP-8/TIMP-1 and MMP-8/TIMP-2 ratios were also found, differences between other ratios before and after therapy were insignificant.
Conclusions
Metalloproteinases and their inhibitors, in particular MMP-9 and TIMP-2, are valuable markers for clinical improvement in RAO.
Keywords: Horse, Lung, Inflammatory marker, Recurrent airway obstruction, Metalloproteinases, Tissue inhibitors of metalloproteinases
Background
Severe equine asthma, commonly known as recurrent airway obstruction (RAO), is a very common pneumopathy in middle-aged to older horses with a prevalence of about 14% in the general horse population [1]. Older studies even found an incidence over 50% of this respiratory disease [2]. Thus, RAO is considered an important problem requiring veterinary attention as it leads to decreased performance capacity and major economic losses [3].
Equine RAO resembles human asthma and COPD in many ways. In all of them, so called remodeling of the pulmonary tissue occurs, which includes reduction of bronchial luminal caliber, smooth muscle hypertrophy, peribronchiolar fibrosis formation and airway epithelial cell hyperplasia, all impeding gas exchange [4, 5]. Control of remodeling may be the key to effective therapy and long-term success of managing these patients [6].
The extracellular matrix (ECM) stabilizes the inner structures of the lung, for which collagen is the most important ECM component. Therefore, the ECM is essential to insure the efficacy of gas exchange. To allow for growth and physiologic tissue repair, it underlies a continuous turnover, but a physiologic balance between synthesis and degradation needs to be maintained. Cleavage of ECM components takes place by zinc-depentant endopeptidases, so called matrix-metalloproteinases (MMPs) and is controlled by their natural inhibitors, the tissue inhibitors of matrix metalloproteinases (TIMPs) [6, 7]. Overwhelming proteolytic degradation in consequence of a dysbalance of MMP and TIMP activity leads to an inflammatory response and ultimately fibrosis formation [8, 9].
Several studies have been published focusing on MMP levels in equine RAO. Increased collagenolytic activity, as indicated by MMP-8 concentrations, was found in tracheal epithelium lining fluid (TELF) [10]. Levels of MMP-8 and MMP-13 were positively correlated with type-I collagen degradation [11].
Markedly increased elastinolytic activity in TELF, indicated by MMP-2 and −9, was also found in RAO [12, 13], but other authors found no disease-associated increase of MMP-2 levels and concluded that its major role may be the physiologic ECM turnover [14]. MMP-9 on the other hand shows very low levels in health, but increases dramatically during exacerbations of asthma and RAO as well as in idiopathic pulmonary fibrosis [15].
While levels of MMPs have been studied intensively in RAO, not much is known about their natural inhibitors, in particular TIMP-1 and TIMP-2, as well as the course of MMPs and TIMPs under different forms of therapy. The inhibitory effect of different tetracyclines, flunixin meglumine and pentoxifylline on elastinolytic activity after intravenous infusion of lipopolysaccharide (LPS) was evaluated [16]. All MMP inhibitors significantly decreased MMP activities, but pentoxifylline and oxytetracycline appeared to be the most effective. Collagenase activity also could be reduced by doxycycline inhibition in tracheal aspirates [10]. These authors concluded that MMP inhibitors might be a valuable new treatment approach to equine RAO. A similar effect was found in a clinical trial in human medicine, in which COPD patients with stable symptoms showed significant improvement in lung function after 4-weeks of doxycycline treatment [17].
Before testing MMP inhibitors in a clinical setting, we were interested in the course of MMPs and TIMPs under the cornerstone of RAO therapy, namely environmental dust reduction in combination with anti-inflammatory glucocorticoids, which were given in an inhalative approach to achieve high local concentrations in the lung. The focus of this study was the evaluation of possible correlations between clinical and cytological parameters with MMP-2, −8 and −9 as well as TIMP1- and −2 activities and concentrations before and after 10 days of inhalative budesonide therapy and environmental dust reduction (EDR).
Methods
Preparticipation examination
10 horses (4 geldings, 6 mares, age 16.5 ± 4.3 years, BDW 481 ± 80.4 kg) with a history of RAO were examined to evaluate the current disease status using a validated clinical score system, recommended by an international workshop [18, 19] and modified by including cytology of BALF instead of tracheal aspirates [20], the previous diagnosis was confirmed (Table 1). Horses in remission, not meeting the inclusion criteria for RAO exacerbation, but still showing parameters above reference values, were included into the study, as these patients account for a large part of our hospital population and often show further improvement under therapy.
Table 1.
Clinical scoring system, modified from Ohnesorge et al. (1998): The highest score given in each subcategory counts as maximum points for this subcategory, maximum points of subcategories are summed up to gain the total score number
| Score | Max. Points | ||
|---|---|---|---|
| 1. Coughing | No cough after manual compression of larynx | 0 | 1 |
| from history, spontaneously or induced | 1 | ||
| 2. Dyspnoea at rest | moderately increased abdominal effort | 1 | 3 |
| Nostril flare | 3 | ||
| Hypertrophy of abdominal muscles | 3 | ||
| 3. Percussion lung field | >1 hand increase | 1 | 2 |
| >2 hands increase | 2 | ||
| 4. Lung auscultation | Wheezes and crackles | 2 | 2 |
| 5. Endoscopy | Significantly increased secretions with moderate viscosity | 1 | 2 |
| Highly increased secretions with high viscosity | 2 | ||
| Marked thickening of tracheal bifurcation [52] | 1 | ||
| 6. BALF cytology | Neutrophils <8% | 0 | 3 |
| Neutrophils 8–15% | 1 | ||
| Neutrophils 15–25% | 2 | ||
| Neutrophils >25% | 3 | ||
| 7. Blood gas analysis | PAO2-PaO2: 0–7 mmHg | 0 | 2 |
| PAO2-PaO2: 7–14 mmHg | 1 | ||
| PAO2-PaO2: >14 mmHg | 2 | ||
BALF collection and processing
Under local anesthesia using 20 ml of 2% lidocaine1 applied to the main bronchi, bronchoalveolar lavage was performed using a silicone catheter.2 Five hundred milliliters of pre-warmed phosphate buffered saline3 were infused and immediately aspirated. BALF was divided into 2 aliquots for cytological and biochemical examination. After centrifugation4 (250 g, 10 min at 4 °C) the BALF supernatant was kept at −80 °C until further analysis. Cytology was performed using Wright-Giemsa staining and counting 500 cells at 500x magnification.
Gelatine zymography of elastases
Zymography was performed5 , 6 following the manufacturer’s instructions. Human MMP-2 and MMP-9 controls were used together with a multicolor broad protein range protein ladder as a control on each gel. Also, a sample of a healthy control horse (samples of a previous study) was applied to each gel to compare the signals to affected horses. Gels were scanned for digital analysis by densitometry using digital image analyzing software7 to quantify the bands objectively as described previously [21].
ELISA of MMPs and TIMPs
Commercially available ELISA systems were used for quantification of MMP-2, MMP-9, TIMP-1 and TIMP-2 concentrations.8 9 10 11 Duplicate standards and samples were measured following the manufacturer’s instructions and absorbance measured with an ELISA microplate reader12 at 450 nm.
Fluorimetry MMP-8 assay
For the MMP-8 assay13 the manufacturer’s instructions were followed. Negative controls containing assay buffer and positive controls using recombinant human purified MMP-8 were included.
Inhalation therapy
Inhalation therapy was performed twice daily at a dosage of 1500 μg budesonide (Pulmicort™ suspension)14 using an automatic inhalation device15. Although the study participants had been under environmental dust reduction (wood shavings as bedding and wet hay as roughage) at their home stables prior to the study, they were stabled in the clinic for overall 16 days to ensure the same conditions concerning stabling and application of inhalation therapy for all animals. During the phase of inhalation therapy a daily clinical examination ensured that no horse was suffering from complications or showed clinical signs of respiratory infection.
Check-up examination
After 10 days of inhalation therapy, the horses were examined following the same protocol as for the preparticipation examination exclusive of thoracic radiography. Afterwards, they were discharged from the clinic with instructions to the owners concerning further management and therapy.
Statistical analysis
Statistical analysis was performed using SPSS Statistics16 and results were expressed as mean ± standard deviation (SD). As parts of data were not found to be normally distributed using the Shapiro Wilks Test, non-parametric tests were used for the entire analysis. The level of significance was set at P < 0.05.
Spearman rank correlation coefficients were calculated between clinical and cytological parameters, MMP and TIMP concentrations or activities, respectively. The level of significance was set at P < 0.05.
The Wilcoxon signed ranks test was used to analyze differences before and after budesonide inhalation in combination with environmental dust reduction Again, the level of significance was set at P < 0.05.
Results
Clinical scoring
The anamnestic diagnosis of RAO was confirmed for all 10 horses. 7 horses were classified RAO in exacerbation, 3 in partial remission. All were subjected to treatment. The total examination score before and after therapy showed a significant difference before and after therapy (P = 0.005) with the endoscopy score being the single parameter of highest significance (P = 0.007).
Inhalation therapy and environmental dust reduction resulted in significantly decreased neutrophil ratio after therapy (25.7 ± 19.4%) compared to before therapy (42.92 ± 27%). As neutrophils decreased (P = 0.013), the relative percentage of macrophages increased (P = 0.022) from 32.77 ± 18.7% before therapy to 45 ± 16.57% after therapy.
Results of clinical scoring are shown in Table 2.
Table 2.
Mean results ± standard deviation (Min-Max) of clinical scoring before and after therapy in 10 RAO horses
| Parameter | Before therapy | After therapy | P |
|---|---|---|---|
| Coughing | 0.5 ± 0.53 (0–1) |
0.3 ± 0.48 (0–1) |
0.157 |
| Dyspnea at rest | 1.1 ± 1.1 (0–3) |
0.7 ± 0.82 (0–2) |
0.046* |
| Percussion of the lung field | 0 ± 0 (0–0) |
0 ± 0 (0–0) |
1 |
| Lung auscultation | 0 ± 0 (0–0) |
0 ± 0 (0–0) |
1 |
| Endoscopy | 1.5 ± 0.53 (1–2) |
0.6 ± 0.52 (0–1) |
0.007* |
| BALF cytology | 2.4 ± 1.1 (0–3) |
2 ± 1.25 (0–3) |
0.157 |
| Blood gas analysis | 0.4 ± 0.7 (0–2) |
0 ± 0 (0–0) |
0.102 |
| Total clinical score | 6 ± 2.6 (2–10) |
3.1 ± 2*↓
(0–5) |
0.005* |
* marks significance at P < 0.05
MMP-2 ELISA
The concentration of MMP-2 was significantly decreased after therapy (3.54 pg/ml ± 0.71 pg/ml) compared to before therapy (4.93 pg/ml ± 0.91 pg/ml, P = 0.005). MMP-2 activity in BALF detected by gelatin zymography was significantly decreased after therapy (5827.45 ± 5250.42) compared to that before therapy (12570.98 ± 9164.32, P = 0.028).
MMP-9 ELISA
MMP-9 concentration in BAL was significantly decreased after therapy (282.29 ng/ml ± 101.19 ng/ml) compared to before therapy (414.59 ng/ml ± 119.6 ng/ml, P = 0.005). The MMP-9 activity in gelatin zymography was significantly decreased after therapy (2942.95 ± 2440.24) compared to before therapy (11176.68 ± 11255.3, P = 0.022).
Examples of MMP-2 and MMP-9 zymography before and after therapy are shown in Figs. 1a and b.
Fig. 1.
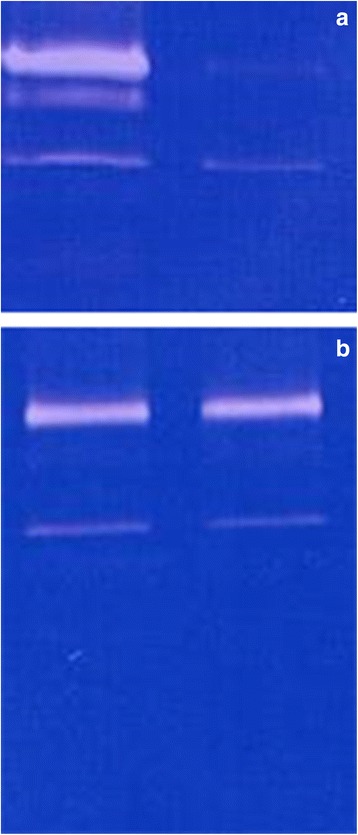
Zymography of MMP-2 (lower band) and MMP-9 (upper band) before (left) and after (right) inhalation therapy in a horse showing clear improvement in clinical and cytologic data (a) versus a horse, in which therapy did not lead to significant improvement (b)
TIMP-1 ELISA
TIMP-1 concentration was significantly decreased after therapy (249.78 ± 59.56 pg/ml) compared to before therapy (308.80 ± 7.44 pg/ml, P = 0.005).
TIMP-2 ELISA
TIMP-2 concentration was also significantly decreased after therapy (18.76 ± 2.79 ng/ml) compared to before therapy (26.20 ± 1.49 ng/ml, P = 0.002).
MMP-8 fluorimetry
The activity of MMP-8 was significantly decreased after therapy (0.10 RFU ± 0.11 RFU) compared to before therapy (0.57 RFU ± 0.85 RFU, P = 0.005).
Results of MMP- and TIMP-measurements are presented in Table 3.
Table 3.
MMP-2, MMP-9, TIMP-1 and TIMP-2 ELISA and MMP-8 fluorimetry measurements, the results are expressed as mean ± SD
| n | Before therapy | After therapy | P-value | |
|---|---|---|---|---|
| MMP-2 ELISA [pg/ml] | 10 | 4.93 ± 0.91 | 3.54 ± 0.71 | 0.005 |
| MMP-9 ELISA [ng/ml] | 10 | 414.59 ± 119.6 | 282.29 ± 101.19 | 0.005 |
| TIMP-1 ELISA [pg/ml] | 10 | 308.80 ± 7.44 | 249.78 ± 59.56 | 0.005 |
| TIMP-2 ELISA [ng/ml] | 10 | 26.20 ± 1.49 | 18.76 ± 2.79 | 0.002 |
| MMP-8 Fluorimetry [RFU] | 10 | 0.57 ± 0.85 | 0.1 ± 0.11 | 0.005 |
| MMP-2 zymography | 10 | 12570.98 ± 9164.32 | 5827.45 ± 5250.42 | 0.028 |
| MMP-9 zymography | 10 | 11176.68 ± 11255.3 | 2942.95 ± 2440.24 | 0.022 |
Correlation of clinical score and MMPs/TIMPs
Strong positive correlations were found between the total clinical scoring and concentrations of MMP-2 (r = 0.66), MMP-9 (r = 0.83), TIMP-1 (r = 0.65), TIMP-2 (r = 0.72) concentrations, MMP-8 activity (r = 0.75) and weakly for MMP-2 (r = 0.47) activity measured by gelatin zymography. All these correlations had a level of significance of P < 0.01, except MMP-2 zymography (P = 0.036). No significant correlation was found between the clinical examination score and MMP-9 zymography (r = 0.30).
Correlations of BALF cytology and MMPs/TIMPs
BAL neutrophil percentages showed no correlation with the concentrations of MMP-2 (r = 0.39), but significant correlations with MMP-9 (r = 0.59), TIMP-1 (r = 0.65), TIMP-2 (r = 0.72) concentrations and MMP-8 activity (r = 0.78) with P < 0.01. MMP-2 activity measured by gelatin zymography was also weakly correlated (r = 0.45), while MMP-9 (r = 0.25) activity was not (P < 0.05).
MMP/TIMP ratios
Significant differences were found for MMP-8 / TIMP-1 (P = 0.002) and MMP-8 / TIMP-2 (P = 0.038) ratios before and after therapy as shown in Table 4.
Table 4.
MMP/TIMP ratios before and after therapy (n = 10)
| MMP-2/ TIMP-1 | MMP-2/ TIMP 2 | MMP-9/ TIMP-1 | MMP-9/ TIMP-2 | MMP-8/ TIMP-1 | MMP-8/ TIMP-2 | |
|---|---|---|---|---|---|---|
| Before therapy | 0.016 | 0.188 | 1.343 | 15.824 | 0.002 | 0.022 |
| After therapy | 0.014 | 0.188 | 1.130 | 15.047 | 0.000* | 0.005* |
The (*) shows significance at P < 0.05
Discussion
Pathogenesis of airway remodeling – In human asthma, activation of fibroblasts leads to increases of ECM mass by overwhelming fibrosis formation, a process similar to bronchial remodeling in equine RAO. This is suspected to be the consequence of a pathologic dysbalance between proteolytic degradation of ECM components by MMPs, and excessive tissue repair primarily mediated by TIMP-1, which can be upregulated very effectively and is capable of cleaving all active MMPs. Elastinolytic and collagenolytic activity was found in several studies on human asthma, in particular increased levels of MMP-9 [22, 23], but also MMP-2 [24, 25]. Former studies of our group showed that this is also true for several equine pneumopathies including RAO, inflammatory airway disease (IAD) and chronic interstitial pneumopathy compared to healthy controls [26, 27]. Compared to these controls, all horses of the presented study showed increased levels of MMP 2, 8 and 9 prior to therapy. Increased TIMP levels have also been found in humans suffering from asthma [23, 24, 28, 29] and the same was found for equine RAO in this study and previous [26, 27]. This may allow the suspicion that fibrosis formation as a long-term consequence of human asthma and equine RAO may be the ultimate result of continuous activation of tissue repair mechanisms or over-repair processes [30]. The most important source of MMP-8, MMP-9 and TIMP-1 are neutrophils, which are typically increased in RAO, accounting for more than 25% of BALF cytology by definition [1]. Therefore, decreases of these enzymes in accordance with decreasing percentages of neutrophils can be expected.
Effects of corticosteroids on MMPs and TIMPs - According to the GINA (global initiative for asthma) guidelines, inhalative therapy using various corticosteroids including budesonide is the key factor of asthma therapy after the control of acute disease exacerbation and is commonly used for persistent long-term management [31]. Reduced pulmonary function and increased inflammatory mediators are also found in equine RAO during phases of clinical remission [32] and corticosteroid treatment in combination with ß2-mimetic bronchodilatators and secretolytic therapy is accepted as the most successful therapy after consequent environmental dust-control [1]. Inhalative treatment is becoming more and more popular in equine medicine and results may be more comparable to what is known in asthmatic patients, so we chose this route of application for our study. Glucocorticoids were shown to slow down the process of bronchial remodeling by downregulation of elastinolytic activity and TIMP upregulation at the same time [33], but other authors could not confirm this observation. Data of the presented study shows indeed the most significant decrease in MMP-9 compared to MMP-2 and 8, but there were also significant decreases in TIMP-1 and TIMP-2. Ex vivo studies demonstrated the ability of corticosteroids to decrease MMP-9 and TIMP-1 levels [34]. In human lung fibrosis, MMP-9 expression could be inhibited with steroids and immunosuppressants [35]. Fibrosis formation is also a long-term effect of equine RAO [36, 37] and it might be suspected that TIMPs decrease in reaction to decreasing MMPs. Nevertheless, ratios of elastases and TIMPs did not differ before and after inhalative therapy in combination with environmental dust control in the presented study.
Todorova et al. [30] studied the effect of inhaled budesonide combined with the bronchodilator formoterol on the metalloproteolytic balance between MMPs and TIMPs. This combination therapy inhibited upregulation of prostaglandine and TIMP-1 production, MMP-9 levels and the MMP-9 / TIMP-1 ratio in human lung fibroblasts, whereas MMP-2 was not affected. Budesonide monotherapy required higher concentrations and formoterol alone had no effects. Therefore, the synergistic effect of budesonide and formoterol was considered the best option to reduce enhanced metalloproteolytic activity and may inhibit or at least reduce pulmonary fibrosis formation in asthma. We might have enhanced the effect of our therapy on MMPs in horses, if the budesonide had been combined with a ß2-agonist like clenbuterol, which is commonly used in horses. This should be the subject of further studies. In asthmatic rats sensitized by ovalbumin and challenged with mist inhalation, steroid treatment not only reduced MMP-9 and TIMP-1 levels, but also improved hyperplasia of airway smooth muscle and basement membrane [38]. Inhalation of budesonide only influences MMP-9 and TIMP-1 levels in moderate to severe disease though, no effect of inhaled budesonide was found in subjects suffering from mild asthma [25].
Obase et al. [39] studied the effects of inhaled budesonide in children with mild and moderate symptoms of asthma on MMP-8 and TIMP-1 levels in induced sputum. Before the beginning of inhalation, MMP-8 was higher in asthma of moderate severity, TIMP-1 was lower and the MMP-8/TIMP-1 ratio was higher in mild and moderate asthma in comparison to healthy childeren. TIMP-1 levels increased in consequence of budesonide inhalation leading to normalization of the the MMP-8 / TIMP-1 ratio. Nevertheless, the excessive MMP-8 levels remained in the airways of children with moderate symptoms. Therefore, corticosteroids may be capable of controlling asthma symptoms, but may be insufficient in preventing the occurrence of airway remodeling involving MMP-8. Results of the presented study, however, show a significant reduction in MMP-8 activity after corticosteroid therapy, although most horses were in disease exacerbation before starting the inhalation protocol. There were also significant reductions in both TIMP concentrations as well as in the MMP-8 / TIMP-1 and the MMP-8 / TIMP-2 ratios. This shows that the MMP-8 / TIMP ratios in the horse do not normalize by upregulation of TIMP, but by downregulation of MMP-8. This seems pleasing and would improve prognosis in equine disease compared to human asthma, but further studies should be performed on the role of MMP-8, as airway remodeling is definitely a long-term effect of equine RAO leading to irreversible exercise insufficiency, but fibrosis formation might not be a direct consequence of TIMP overexpression in the horse.
New therapeutic approaches - MMPs themselves have been discussed as an ideal target for new therapeutic approaches as they play an active role in disease pathophysiology [40]. Inhibition of MMP activity has been studied in human cancer. Although this was not successful [41], new data suggests that selective inhibitors might find a role in acute and chronic anti-inflammatory therapy after all [42]. This includes asthma, where reduced airway inflammation and hyperresponsiveness was reported after local bronchial application of synthetic TIMP-2 [43]. TIMP-2 downregulated MMP-2 activity [44]. In addition to their antibiotic acitivity, tetracyclines act as effective MMP inhibitors. Doxycycline was studied in asthma models including different species [10, 45, 46]. After oral administration in mice, it reduced airway inflammation and hyperresponsiveness as well as MMP-9 expression. Further studies have shown that MMP-inhibition also decreases goblet cell hyperplasia, another component of airway remodeling in asthma [47], whichh is also known for equine RAO. In the horse, in-vitro studies showed reductions of proteolytic activity using acetylcysteine, pentamidine and diminazene [14, 48]. The clinical application of synthetic TIMPs in RAO horses is currently under consideration by our group.
Limitations - To our knowledge, this is the first study on the course of MMPs in RAO affected horses and a clinical trial, but we were faced with several limitations, so unintentional bias may have occurred at several points. Due to the fact that privately owned horses were included to the study, it was doubtful if stabling of all horses at their home stables was actually in a low-dust environment, as most horses were still in exacerbation and had increased MMPs at the beginning of therapy. The effect of therapy may therefore be based on the combined effect of inhalative glucocorticoid therapy and environmental dust reduction under clinic conditions, with the effect of the latter being quite variable. Even if horses had been stabled on wood shavings and fed wet hay, they may have come from a very dusty environment apart from these two conditions for enrolment in the study. A second group subjected to a low-dust-environment in the clinic only would have been preferable to control this effect and was planned to be included in the study, but unfortunately, not enough owners agreed to this protocol. Therefore, the actual bias caused by this is hard to assess. Another time-point of sampling after a few days of low-dust-environment before starting inhalation therapy would have been helpful as well, but again, owners wished for hospitalization as short as possible. On the other hand, it was not the aim of this study to prove the therapeutic effect of neither environmental dust reduction, which is commonly acceptected [1] nor inhaled budesonide, which has also already been published [49], but to study the course of MMPs and TIMPs under clinical improvement to evaluate their usefulness as biomarkers and correlation with clinical and cytological findings. Clinical scoring and even the quantitative tests may have been subject to bias as well. The clinical scoring was done by two independent observers, but these were not blinded to horses’enrolment in the study and the time-point as before or after therapy, which was due to the fact that subjects enrolled were clinic patients. Endoscopy and cytology was done by one observer and images scored by another later on randomly and blinded. ELISAs and zymography were performed, when all samples had been collected and stored, also randomly and blinded.
Conclusion
Despite the difficulties discussed above, the results of the presented study suggest that developing new therapeutic approaches to correct the misbalance between MMPs and TIMPs might be promising for treatment of asthma and equine RAO, which shares many features with human asthma disease. Due to their various effects in different organ systems, it seems safer to target MMPs actually relevant in this disease specifically and to use an inhalative approach to reach high local levels of the drug, while the remaining organism remains unaffected. In chronic pneumopathies, targeting MMP-2 and MMP-8, but in particular MMP-9 might be of therapeutic value [50]. First attempts in this direction have been taken in treating cancer, but less data is available on inhibition of MMP activity in chronic pneumopathies [51].
To our knowledge, TIMP-1 or TIMP-2 expression in equine RAO has not been studied so far. Studies in horses on the effect of inhaled glucocorticoids on MMPs or TIMPs have not been published either. Therefore, this is the first clinical study to show a correlation of MMP and TIMP levels to clinical findings and cytology. Parallels to results presented in papers on humans and laboratory animals allow the suspicion that the same mechanisms might apply in the horse regarding the effects of glucocorticoids, but further studies including a control group have to be performed to differentiate a possible therapeutic effect between glucocorticoid inhalation and envirnonmental dust reduction in horses affected by RAO.
Acknowledgements
We thank the owners of the horses for participation in this study.
Funding
This study was funded by a research grant of the Freie Universitaet Berlin.
Availability of data and materials
Original datasets are available for review on request.
Authors contributions
AKB carried out the clinical examinations, participated in the conceivement, design and coordination of the study and drafted the manuscript. TS carried out the laboratory assays and performed the statistical analysis. AB participated in the coordination of the study and participated in the laboratory assays, RE participated in the coordination of the study and laboratory assays, HG conceived of the study, participated in its design and coordination. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval
Sampling of horses affected by respiratory disease was not classified as animal experiments by the State Office of Health and Social Affairs Berlin (LaGeSo). The owners gave permission to involve their horses in the study.
Abbreviations
- AaDo2
Arterio-alveolar pressure difference
- BALF
Bronchoalveolar lavage fluid
- BDW
Body weight
- BGA
Blood gas analysis
- BID
Bis in die, twice daily
- CIP
Chronic interstitial pneumopathy
- COPD
Chronic obstructive pulmonary disease
- ECM
Extracellular matrix
- EDR
Environmental dust reduction
- ELISA
Enzyme-linked immunosorbent assay
- GINA
Global initiative for asthma
- IAD
Inflammatory airway disease
- LaGeSo
State Office of Health and Social Affairs Berlin
- LPS
Lipopolysaccharide
- MMP
Matrix metalloproteinase
- PaO2
Partial oxygen pressure
- RAO
Recurrent airway obstruction
- Rpm
Rounds per minute
- SD
Standard deviation
- TELF
Tracheal epithelium lining fluid
- TIMP
Tissue inhibitor of metalloproteinases
Footnotes
Lidocaine, bela-Pharm GmbH, Vechta, Germany
Silicone Bronchoalveolar Lavage Catheter 300 cm, Smiths Medical ASD, Inc, USA
Phosphate buffered saline, Lonza, Verviers, Belgium;dTable Top Refrigerated Centrifuge Hermle Z326K, Hermle Labortechnik GmbH, Germany
Gelatin zymogram gels, Life technologies, USA
electrophoresis device XCell, Novex Experimental Technology, Japan
ImageJ v1.47, Wayne Rasband, NIH, USA
equine MMP-2 kit, USCN Life Science Inc., China
equine MMP-2 kit, USCN Life Science Inc., China
equine MMP-9 kit, USCN Life Science Inc., China
equine TIMP-1 kit, USCN Life Science Inc., China
equine TIMP-2 kit, USCN Life Science Inc., China
ELISA microplate reader, BioRad Laboratories, USA
SensolyteTM 520 MMP-8 Assay Kit, Anaspec, Inc. Fermont, USA
Pulmicort™ suspension, Astra Zeneca, Germany
SaHoMa II mobile ultrasonic nebulizer, NEBU-TEC International, Germany
SPSS Statistics, Version 17.0 released 2008, SPSS Inc., USA
Contributor Information
Ann Kristin Barton, Email: Ann-Kristin.Barton@fu-berlin.de.
Tarek Shety, Email: Tarek.Shety@gmail.com.
Angelika Bondzio, Email: Angelika.Bondzio@fu-berlin.de.
Ralf Einspanier, Email: Ralf.Einspanier@fu-berlin.de.
Heidrun Gehlen, Email: Heidrun.Gehlen@fu-berlin.de.
References
- 1.Pirie RS. Recurrent airway obstruction: a review. Equine Vet J. 2014;46:276–288. doi: 10.1111/evj.12204. [DOI] [PubMed] [Google Scholar]
- 2.Bracher V, von Fellenberg R, Winder CN, et al. An investigation of the incidence of chronic obstructive pulmonary disease (COPD) in random populations of Swiss horses. Equine Vet J. 1991;23:136–141. doi: 10.1111/j.2042-3306.1991.tb02738.x. [DOI] [PubMed] [Google Scholar]
- 3.Roy MF, Lavoie JP. Tools for the diagnosis of equine respiratory disorders. Vet Clin North Am Equine Pract. 2003;19:1–17. doi: 10.1016/S0749-0739(02)00063-9. [DOI] [PubMed] [Google Scholar]
- 4.Lavoie JP. Recurrent Airway Obstruction (Heaves) and Summer-pasture-associated Obstructive Pulmonary Disease. In: McGorum BC, Dixon PM, Robinson NE, Schumacher J, editors. Equine Respiratory Medicine and Surgery. Edinburgh: W.B. Saunders; 2007. pp. 565–590. [Google Scholar]
- 5.Lugo J, Harkema JR, deFeijter-Rupp H, et al. Airway inflammation is associated with mucous cell metaplasia and increased intraepithelial stored mucosubstances in horses. Vet J. 2006;172:293–301. doi: 10.1016/j.tvjl.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Lu P, Takai K, Weaver VM et al. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011; 3: doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed]
- 7.Clutterbuck AL, Harris P, Allaway D, et al. Matrix metalloproteinases in inflammatory pathologies of the horse. Vet J. 2010;183:27–38. doi: 10.1016/j.tvjl.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman HA. Disorders of lung matrix remodeling. J Clin Invest. 2004;113:148–57. doi: 10.1172/JCI20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koivunen AL, Maisi P, Konttinen YT, et al. Collagenolytic activity and its sensitivity to doxycycline inhibition in tracheal aspirates of horses with chronic obstructive pulmonary disease. Acta Vet Scand. 1997;38:9–16. doi: 10.1186/BF03548503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raulo SM, Sorsa TA, Kiili MT, et al. Evaluation of collagenase activity, matrix metalloproteinase-8, and matrix metalloproteinase-13 in horses with chronic obstructive pulmonary disease. Am J Vet Res. 2001;62:1142–8. doi: 10.2460/ajvr.2001.62.1142. [DOI] [PubMed] [Google Scholar]
- 12.Raulo SM, Sorsa TA, Tervahartiala T, et al. MMP-9 as a marker of inflammation in tracheal epithelial lining fluid (TELF) and in bronchoalveolar fluid (BALF) of COPD horses. Equine Vet J. 2001;33:128–36. doi: 10.1111/j.2042-3306.2001.tb00590.x. [DOI] [PubMed] [Google Scholar]
- 13.Raulo SM, Sorsa TA, Maisi PS. Concentrations of elastinolytic metalloproteinases in respiratory tract secretions of healthy horses and horses with chronic obstructive pulmonary disease. Am J Vet Res. 2000;61:1067–1073. doi: 10.2460/ajvr.2000.61.1067. [DOI] [PubMed] [Google Scholar]
- 14.Koivunen AL, Maisi P, Konttinen YT, et al. Gelatinolytic activity in tracheal aspirates of horses with chronic obstructive pulmonary disease. Acta Vet Scand. 1997;38:17–27. doi: 10.1186/BF03548504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol. 2003;28:12–24. doi: 10.1165/rcmb.2002-0166TR. [DOI] [PubMed] [Google Scholar]
- 16.Fugler LA, Eades SC, Moore RM, et al. Plasma matrix metalloproteinase activity in horses after intravenous infusion of lipopolysaccharide and treatment with matrix metalloproteinase inhibitors. Am J Vet Res. 2013;74:473–480. doi: 10.2460/ajvr.74.3.473. [DOI] [PubMed] [Google Scholar]
- 17.Dalvi PS, Singh A, Trivedi HR, et al. Effect of doxycycline in patients of moderate to severe chronic obstructive pulmonary disease with stable symptoms. Ann Thorac Med. 2011;6:221–226. doi: 10.4103/1817-1737.84777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohnesorge B, Trötschel C and Deegen E. Diagnostic value of capnography in horses with RAO. Proceedings 5th World Equine Vet Assoc Congress 1998, p. 65–69.
- 19.Robinson NE. International workshop on equine chronic airway disease, Michigan State University 16–18 June 2000. Equine Vet J. 2001;33:5–19. doi: 10.2746/042516401776767412. [DOI] [PubMed] [Google Scholar]
- 20.Dieckmann M, Deegen E. Clinical aspect of tracheobronchial aspirates cytology. Pferdeheilkunde. 1990;6:101–110. doi: 10.21836/PEM19900301. [DOI] [Google Scholar]
- 21.Chung MK. Correlation Coefficient. In: Salkind J, editor. Encyclopedia of Measurement and Statistics. London: Sage; 2007. [Google Scholar]
- 22.Ohbayashi H, Shimokata K. Matrix metalloproteinase-9 and airway remodeling in asthma. Curr Drug Targets Inflamm Allergy. 2005;4:177–181. doi: 10.2174/1568010053586246. [DOI] [PubMed] [Google Scholar]
- 23.Mattos W, Lim S, Russell R, et al. Matrix metalloproteinase-9 expression in asthma: effect of asthma severity, allergen challenge, and inhaled corticosteroids. Chest. 2002;122:1543–1552. doi: 10.1378/chest.122.5.1543. [DOI] [PubMed] [Google Scholar]
- 24.Cataldo D, Munaut C, Noel A, et al. MMP-2-and MMP-9-linked gelatinolytic activity in the sputum from patients with asthma and chronic obstructive pulmonary disease. Int Arch Allergy Immunol. 2000;123:259–267. doi: 10.1159/000024452. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki R, Kato T, Miyazaki Y, et al. Matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in sputum from patients with bronchial asthma. J Asthma. 2001;38:477–484. doi: 10.1081/JAS-100105868. [DOI] [PubMed] [Google Scholar]
- 26.Barton A, Shety T, Pelli A, et al. Evaluation of inflammatory markers and treatment success in inflammatory airway disease under the course of inhalation therapy and environmental dust reduction. Cabourg: Proceedings 2nd Havemeyer Workshop on IAD; 2014. [Google Scholar]
- 27.Barton A, Shety T, Bondzio A, et al. Metalloproteinases and their tissue inhibitors in comparison between different chronic pneumopathies in the horse, Med Inflamm. 2015, http://dx.doi.org/10.1155/2015/569512 [DOI] [PMC free article] [PubMed]
- 28.Vignola AM, Riccobono L, Mirabella A, et al. Sputum metalloproteinase-9/tissue inhibitor of metalloproteinase-1 ratio correlates with airflow obstruction in asthma and chronic bronchitis. Am J Respir Crit Care Med. 1998;158:1945–1950. doi: 10.1164/ajrccm.158.6.9803014. [DOI] [PubMed] [Google Scholar]
- 29.Mautino G, Henriquet C, Jaffuel D, et al. Tissue inhibitor of metalloproteinase-1 levels in bronchoalveolar lavage fluid from asthmatic subjects. Am J Respir Crit Care Med. 1999;160:324–330. doi: 10.1164/ajrccm.160.1.9808087. [DOI] [PubMed] [Google Scholar]
- 30.Todorova L, Gürcan E, Westergren-Thorsson G, et al. Budesonide/ formoterol effects on metalloproteolytic balance in TGFb-activated human lung fibroblasts. Respir Med. 2009;103:1755–1763. doi: 10.1016/j.rmed.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Global Initiative for Asthma (GINA). Global strategy for asthma managementand prevention. NIH Publication 02–3659 issued January 1995 (updated 2004).
- 32.Lavoie-Lamoureux A, Leclere M, Lemos K, et al. Markers of systemic inflammation in horses with heaves. J Vet Intern Med. 2012;26:1419–1426. doi: 10.1111/j.1939-1676.2012.00993.x. [DOI] [PubMed] [Google Scholar]
- 33.Hoshino M, Takahashi M, Takai Y, et al. Inhaled corticosteroids decrease subepithelial collagen deposition by modulation of the balance between matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 expression in asthma. J Allergy Clin Immunol. 1999;104:356–363. doi: 10.1016/S0091-6749(99)70379-9. [DOI] [PubMed] [Google Scholar]
- 34.Profita M, Gagliardo R, Di Giorgi R, et al. In vitro effects of flunisolide on MMP-9, TIMP-1, fibronectin, TGF-beta1 release and apoptosis in sputum cells freshly isolated from mild to moderate asthmatics. Allergy. 2004;59:927–932. doi: 10.1111/j.1398-9995.2004.00516.x. [DOI] [PubMed] [Google Scholar]
- 35.Gueders MM, Foidart JM, Noel A, et al. Matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs in the respiratory tract: potential implications in asthma and other lung diseases. Eur J Pharmacol. 2006;533:133–144. doi: 10.1016/j.ejphar.2005.12.082. [DOI] [PubMed] [Google Scholar]
- 36.Kaup FJ, Drommer W, Deegen E. Ultrastructural findings in horses with chronic obstructive pulmonary-disease (COPD). 1. Alterations of the larger conducting airways. Equine Vet J. 1990;22:343–348. doi: 10.1111/j.2042-3306.1990.tb04287.x. [DOI] [PubMed] [Google Scholar]
- 37.Kaup FJ, Drommer W, Damsch S, et al. Ultrastructural findings in horses with chronic obstructive pulmonary disease (COPD) II: Pathomorphological changes of the terminal airways and the alveolar region. Equine Vet J. 1990;22:349–355. doi: 10.1111/j.2042-3306.1990.tb04288.x. [DOI] [PubMed] [Google Scholar]
- 38.Qiao HM, Lu JR, Cheng HJ, et al. Regulatory effects of inhaled steroids on expression of matrix metalloproteinase-9 and its inhibitor in asthmatic rats. Zhonghua Er Ke Za Zhi. 2005;43:591–594. [PubMed] [Google Scholar]
- 39.Obase Y, Rytilä P, Metso T, et al. Effects of Inhaled Corticosteroids on Metalloproteinase-8 and Tissue Inhibitor of Metalloproteinase-1 in the Airways of Asthmatic Children. Int Arch Allergy Immunol. 2010;151:247–254. doi: 10.1159/000242362. [DOI] [PubMed] [Google Scholar]
- 40.Fingleton B. MMPs as therapeutic targets--still a viable option? Semin Cell Dev Biol. 2008;19:61–68. doi: 10.1016/j.semcdb.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vihinen P, Ala-aho R, Kahari VM. Matrix metalloproteinases as therapeutic targets in cancer. Curr Cancer Drug Targets. 2005;5:203–220. doi: 10.2174/1568009053765799. [DOI] [PubMed] [Google Scholar]
- 42.Hu J, Van den Steen PE, Sang QXA, et al. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6:480–498. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- 43.Kumagai K, Ohno I, Okada S, et al. Inhibition of matrix metalloproteinases prevents allergen-induced airway inflammation in a murine model of asthma. Am J Respir Crit Care Med. 1999;159:325. [PubMed] [Google Scholar]
- 44.Kumagai K, Ohno I, Imai K, et al. The involvement of matrix metalloproteinases in basement membrane injury in a murine model of acute allergic airway inflammation. Clin Exp Allergy. 2002;32:1527–1534. doi: 10.1046/j.1365-2745.2002.01491.x. [DOI] [PubMed] [Google Scholar]
- 45.Lee YC, Lee HB, Rhee YK, et al. The involvement of matrix metalloproteinase-9 in airway inflammation of patients with acute asthma. Clin Exp Allergy. 2001;31:1623–1630. doi: 10.1046/j.1365-2222.2001.01211.x. [DOI] [PubMed] [Google Scholar]
- 46.Lee KS, Jin SM, Kim SS, et al. Doxycycline reduces airway inflammation and hyperresponsiveness in a murine model of toluene diisocyanate-induced asthma. J Allergy Clin Immunol. 2004;113:902–909. doi: 10.1016/j.jaci.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Yoshisue H, Hasegawa K. Effect of MMP/ADAM inhibitors in goblet cell hyperplasia in cultured human bronchial epithelial cells. Biosci Biotechnol Biochem. 2004;68:2024–2031. doi: 10.1271/bbb.68.2024. [DOI] [PubMed] [Google Scholar]
- 48.Koivunen AL, Maisi P, Fang W, et al. Inhibition of the protease activity in tracheobronchial aspirates of horses with chronic obstructive pulmonary disease. Am J Vet Res. 1996;57:603–607. [PubMed] [Google Scholar]
- 49.Kampmann C, Ohnesorge B, Venner M, et al. Effects of inhaled budesonide on pulmonary mechanics and gas exchange for treatment of COPD. Equine Medicine. 2001;17:155–160. [Google Scholar]
- 50.Kupai K, Szucs G, Cseh S. Matrix metalloproteinase activity assays: Importance of zymography. J Pharmacol Toxicol Methods. 2010;61:205–209. doi: 10.1016/j.vascn.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Ohbayashi H. Matrix metalloproteinases in lung diseases. Curr Protein Pept Sci. 2002;3:409–421. doi: 10.2174/1389203023380549. [DOI] [PubMed] [Google Scholar]
- 52.Gerber V, Straub R, Marti E, et al. Endoscopic scoring of mucus quantity and quality: observer and horse variance and relationship to inflammation, mucus viscoelasticity and volume. Equine Vet J. 2004;36:576–82. doi: 10.2746/0425164044864525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original datasets are available for review on request.


