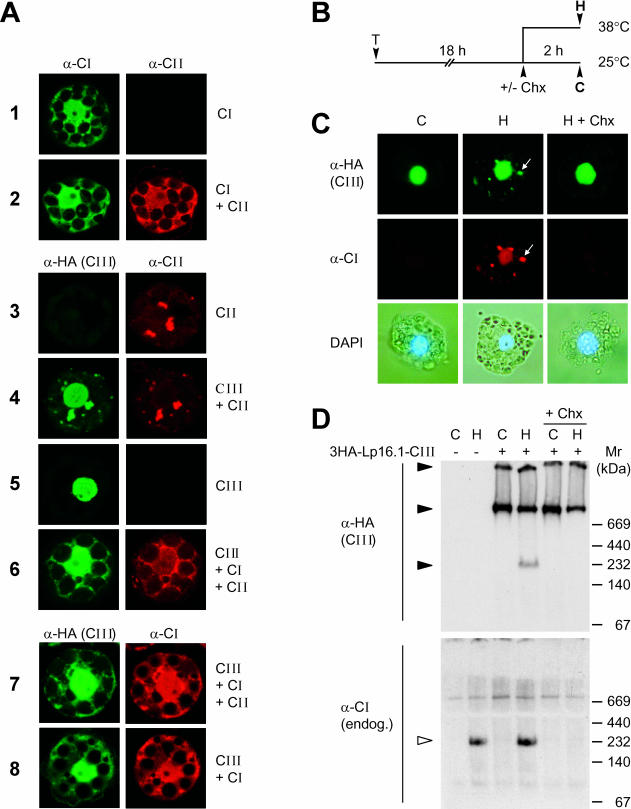
Fig 6.
Expression and intracellular localization of Hsp16.1-CIII in mesophyll protoplasts. (A) Tomato protoplasts were transformed for transient expression of PsHsp18.1-CI (CI), LpHsp17.4-CII (CII), or 3HA-Hsp16.1-CIII (CIII) alone or for coexpression in combinations as indicated for each sample on the right margin. The specific primary antisera used for the immunofluorescence detection of the 3 types of sHsps are indicated on top of the corresponding group of images. (B) Tobacco protoplasts were transformed with plasmid encoding 3HA-LpHsp16.1-CIII. After 18 hours of transgene expression at 25°C, samples were subjected to 2 hours of heat stress at 38°C to induce the endogenous set of chaperones (see pictograph). Cycloheximide (Chx, 5 μg/mL) was added to aliquots of the samples before the heat stress treatment to prevent the formation of endogenous Hsps. (C) Immunofluorescence detection of class CI and class CIII proteins. Arrows point to colocalization of tomato Hsp16.1-CIII with endogenous tobacco Hsp17-CI in cytoplasmic HSG complexes. The lower panel presents pictures of the corresponding cells after nuclear staining with 4′,6-diamidino-2-phenylindole. (D) Analysis of 3HA-Hsp16.1-CIII complexes in nondenaturing gels by immunoblot detection with α-HA (upper part) and α-Hsp17-CI antisera (lower part). Samples corresponding to protoplast transformed with the expression plasmid for 3HA-Hsp16.1-CIII are indicated (+) on top of the gel. Oligomeric complexes are indicated by filled (3HA-Hsp16.1-CIII) and open arrowheads (Hsp17-CI)