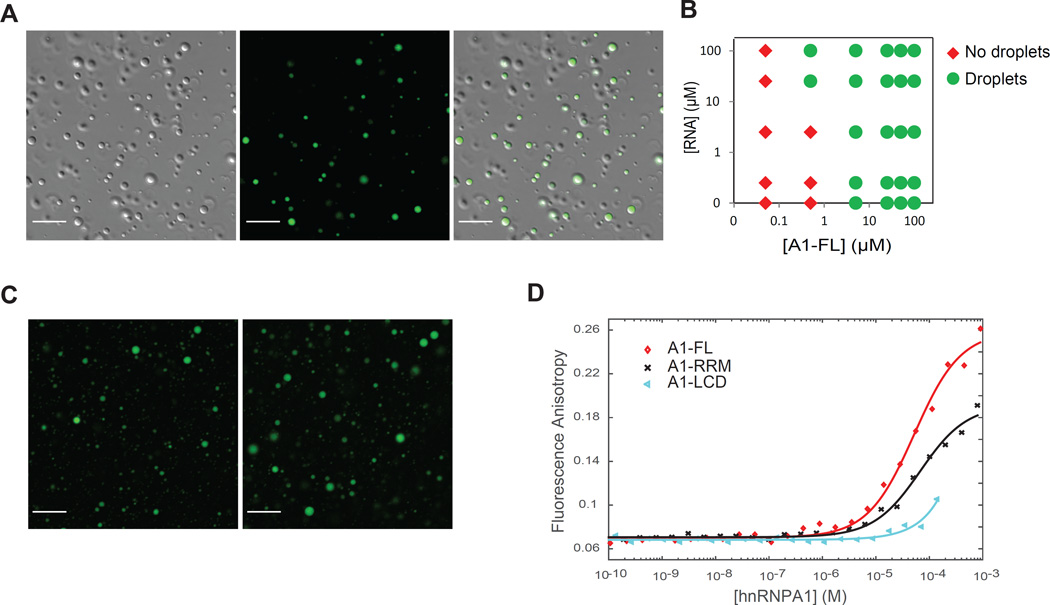
Figure 4. RNA facilitates liquid-liquid phase separation of hnRNPA1 by binding to RRMs and LCD.
(A) Fluorescence images of 120 µM hnRNPA1 (DIC) mixed with 1.2 µM fluorescein-labeled RNA at 10 °C. The samples of purified hnRNPA1 were RNA-free (Figure S1).
(B) Phase diagram of hnRNPA1 as a function of protein concentration and RNA concentration. Red and green symbols indicate that the sample was in the one-phase or the two-phase regime, respectively. The experiment was performed in 50 mM HEPES, 150 mM NaCl, 5 mM DTT and 150 mg/ml Ficoll at 10 °C.
(C) Fluorescence images of 100 µM fluorescein-labeled RNA mixed with 100 µM A1-RRM or A1-LCD at 10 °C.
(D) A1-FL, A1-RRM and A1-LCD binding to RNA was monitored by changes in fluorescence anisotropy of 5’-fluorescein-labeled RNA (fl-RNA44). Symbols represent experimental data points, solid lines are non-linear least squares fits to a direct binding model (Roehrl et al., 2004). Importantly, LLPS did not occur under these conditions; the increase in fluorescence anisotropy is therefore caused by direct binding, not partitioning of RNA into droplets.