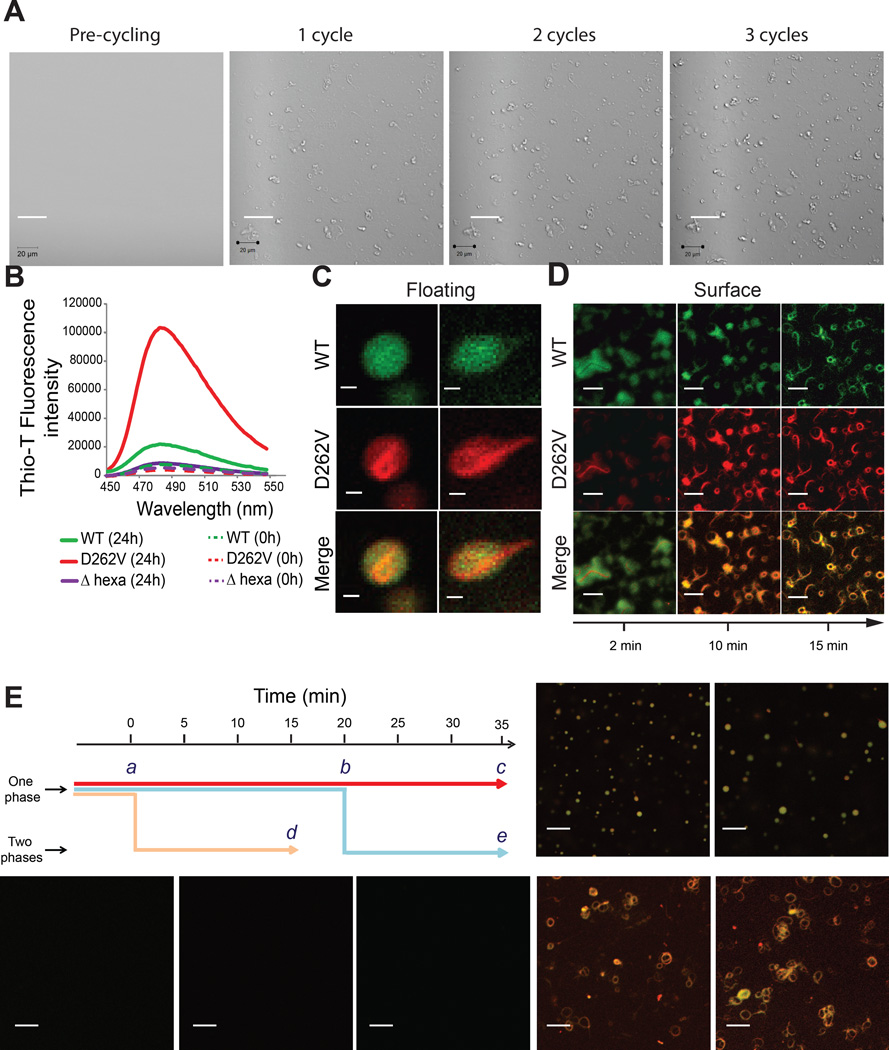
Figure 6. Phase separation promotes fibrillization of hnRNPA1 D262V.
All experiments were performed in 50 mM HEPES, 300 mM NaCl, 5 mM DTT and 100 mg/ml Ficoll.
(A) A1-D262V fibrils accumulation on the surface of the coverslip was monitored by cycling the temperature between 10 °C and 25 °C. Each cycle corresponded to a starting temperature of 25 °C, decreased then to 10 °C to allow droplet formation and increased back to 25 °C. The images were taken at 25 °C in order to visualize the surface. See also Figure S6.
(B) A1-FL, A1-D262V or A1-Δhexa were shaken at 25 °C for 24 h. Fibrillization was monitored by ThT fluorescence.
(C) Fluorescence images of floating droplets of a mixture of Oregon green-labeled/unlabeled wild-type hnRNPA1 (total concentration 160 µM, molar ratio of 1:300) mixed with Rhodamin-Texas red labeled/unlabeled A1 D262V (total concentration 160 µM, molar ratio of 1:300) at 16 °C.
(D) Fluorescence images of a mixture of Oregon green-labeled/unlabeled wild-type hnRNPA1 (total concentration 160 µM, molar ratio of 1:300) mixed with Rhodamin-Texas red labeled/unlabeled A1-D262V (total concentration 160 µM, molar ratio of 1:300) at 33 °C. The images were taken at indicated times at the surface of the coverslips.
(E) Schematic summarizing the experiment to correlate phase separation and fibrillization. The sample was either kept in the one-phase regime (33 °C) for 35 min (red arrow), kept in the one-phase regime for 20 min and then put in the two-phase regime by decreasing the temperature to 16 °C for 15 min (blue arrow) or kept in the two-phase regime for 15 min (yellow arrow). The images were taken at the indicated time points (a–e).