Figure 1.
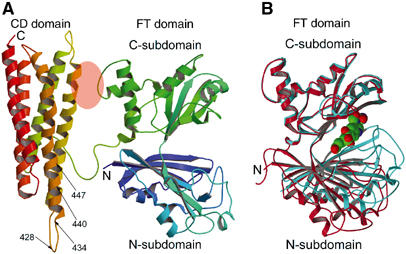
Subunit structure of FTCD. (A) The two-domain (FT and CD) subunit structure of the ligand-free FTCD. Backbone trace color ramped from the N-terminal end (N) to the C-terminal end (C). In this orientation, the lengths of the top and bottom and the two sides are roughly 70 Å and the thickness is about 40 Å. The areas of the FT C-subdomain and CD domain shaded in pink oval are involved in the only interdomain interaction within the subunit, which is mainly between the segment of residues 290–295 of the FT C-subdomain and residues 392–399 of the CD domain (refer also Figure 2). The arrows demarcate the locations of the two major linear antigenic determinants (428NTPEEKD434 and 440LQEGLRRA447) of human FTCD autoantigen (Lapierre et al, 2003), which differ by only one residue from the same segments in rat FTCD (see text). (B) Superpositioning of the C-subdomain of the FT domain in the subunit (red) structure (panel A) with that in the structure of the same domain in isolation (aqua marine) with the product analog, folinic acid (CPK model), bound in the groove (Kohls et al, 2000). The orientation of the FT domain is identical to that shown in panel A. The groove in the ligand-free structure of the intact subunit is wide open, whereas that in the ligand-bound structure of the isolated FT domain is completely closed.
