Figure 6.
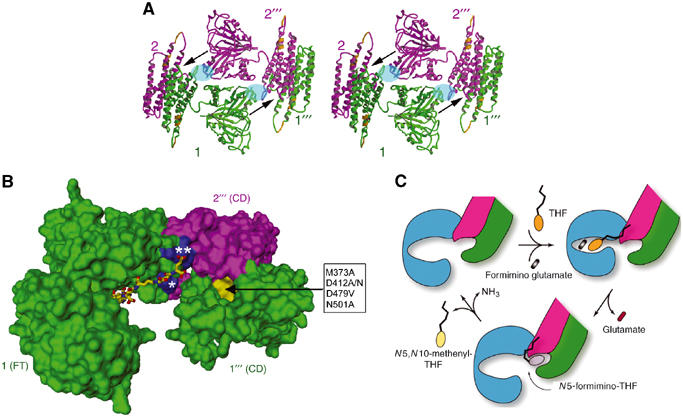
Subunit organization for enzyme activities and intermediate channeling. (A) Stereo view of one side of the D4 octamer. The width of the side is about 140 Å or 14 nm. This view is orthogonal to that shown in Figure 3A. The side is occupied mainly by the isologous stack of two FT domains (e.g., between subunits 1 (green) and 2′′′ (purple)). The FT–FT stack is flanked on both sides by isologous pairs of CD domain helix bundles of subunits 1 and 2 and subunits 2′′′ and 1′′′, which occupy the corners. Each of the arrows indicates the best possible route for channeling the N5-formimino-THF intermediate from the FT catalytic site groove in subunit 1 or 2′′′ to the deaminase catalytic site pocket near the end of a pair of CD domains between subunits 2′′′ and 1′′′ or subunits 1 and 2, respectively. The deep pocket (see also panel B) at each end of the CD−CD pair is the only one found by the POCKET program (see Materials and methods). The local two-fold related areas shaded in blue oval represent regions involved in heterologous interactions, between the FT domain from one subunit and CD domain from another subunit. This interaction is mainly between the N-terminal end (residues 192–198) of helix α6 in the FT C-subdomain and the loop (residues 379–389) connecting helices α14 and α15 in the CD domain (see also Figure 2). The two nearly contiguous segments in the CD domain (gold) represent the equivalent two immunodominant linear epitopes recognized by LC1 autoantibodies (see text and Figure 1A). (B) Geometrical relationship between the FT domain active site groove, the CD active site pocket (yellow surface) and two adjacent patches (blue surface) of positive charge residues. This figure of molecular surface shows only the FT domain from subunit 1 and the two CD domains from subunits 2′′′ and 1′′′. The residues in the box (D479 and M373 from subunit 1′′′ and D412 and N501 from subunit 2′′′) are those in the CD pocket (directed by the arrow) that when mutated resulted in diminution of CD activity (see text and Materials and methods). The FT active site groove is in the open form with the pteroyl moiety of the folinic acid (stick model) bound in the C-subdomain as shown Figure 1B. The ligand has four additional γ-linked glutamates coupled to the glutamate of folinic acid to generate a five-residue polyglutamate tail. Modeling of the polyglutamate tail indicates potential salt links of the two terminal glutamates with highly conserved basic residues (Figure 2), including R/K 193, which lies in the patch (marked with a single asterisk) of subunit 1 FT C-subdomain, and R381 and R382, which lie in the patch (double asterisks) of subunit 2′′′ CD domain. Arg residues (especially doublets such as arginines 381 and 382 in FTCD) have been implicated in the binding of polyglutamylated substrates to folate-dependent enzymes (Finer-Moore et al, 1994; Murley and MacKenzie, 1995). Indeed, we found that mutagenic replacement of Arg 382 to an Ala residue no longer allows FTCD to bind to the polyglutamate affinity column, which is very effective in the purification of the native protein (see Materials and methods). (C) Schematic diagram of the major steps of the dual enzyme activities of FTCD in the D4 quaternary structure. The FT domain is colored blue to emphasize the involvement of three domains (including two CD domains truncated to show only one active site) from three subunits. Starting with the ligand-free FTCD (upper left), the substrates bind in the open form of the FT domain, with the THF (ring structures in orange) initially binding in the C-subdomain and thereby allowing the polyglutamate tail to form salt links with basic residues concentrated at the interface between the FT C-subdomain and CD domain (see panel B). The groove closes (upper right) by movement of the FT N-subdomain toward the C-subdomain, triggering transferase catalysis. The groove then opens (bottom model), thereby releasing the product glutamate and allowing the pteroyl ring (gray) of the N5-formimino-THF intermediate to be shuttled to the CD active site pocket by the swinging motion of the anchored polyglutamate tail for CD catalysis. In the final step, the two products (N5,N10-methenyl-THF (yellow pteridin ring) and ammonia) of the deaminase catalysis are released, and the starting open enzyme form is regenerated.
