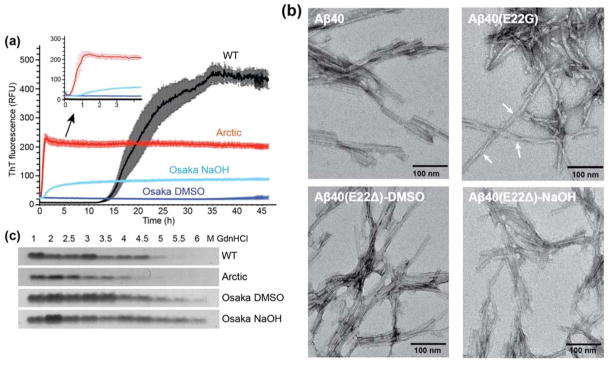
Figure 1.
Fibrillization of wild-type, Arctic and Osaka Aβ40 peptides. (A) Osaka and Arctic Aβ40 peptides form fibrils much more rapidly than the wild-type (WT) peptide. Kinetics of fibril formation (0.2 mg/ml in 10 mM sodium phosphate buffer, pH 7.2) was measured by the amyloid-binding dye ThT (10 μM). n=5, mean±SEM. (B) Negative stain TEM images of WT and mutant Aβ40 fibrils. Arctic Aβ40 fibrils display significant twists with variable periodicities and more pronounced entanglement than wild-type and Osaka fibrils. Scale bar = 100 nm. (C) Mutant fibrils have altered stability to denaturation by GdnHCl. Fibrils were incubated with increasing concentrations (1 – 6 M) of GdnHCl and then ultracentrifuged at 100,000 × g to pellet any fibrils that resisted denaturation, which were resolved by SDS-PAGE and Coomassie stain.