Figure 4.
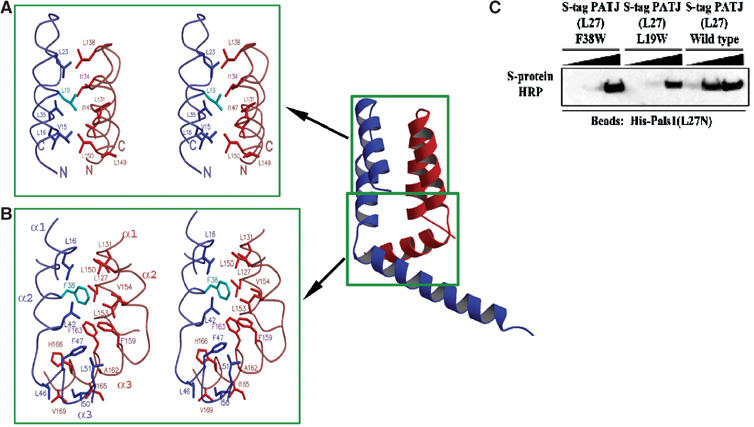
The PALS1–PATJ heterodimer is stabilized by hydrophobic interactions. (A) Stereoview of the hydrophobic packing interactions between the PATJ L27 domain (blue/cyan) and the PALS1 L27N domain (red) focusing on the section of the four-helical bundle closest to the loop that connects the first and second helices of each L27 domain. Mutated residues in PATJ are colored in cyan. (B) Similar to (A), but now the focus is on the interactions in the hydrophobic core formed at the interface of all six helices from both L27 domains. (C) Substituting for a tryptophan at position 19 or 38 of L27PATJ decreases binding to L27PALS1N. Equal amounts of His-L27PALS1N were immobilized on Ni-NTA agarose beads. The lysate from cells expressing wild-type S-tag-L27PATJ or the indicated mutated proteins was added to these beads (5, 50, or 500 μl diluted to 500 μl) and incubated for 2 h at 4°C. Beads were then washed three times in HNTG. Sample buffer was then added and precipitates were subjected to SDS–PAGE and transferred to nitrocellulose. S-tagged proteins were visualized via immunoblotting with S-protein HRP.
