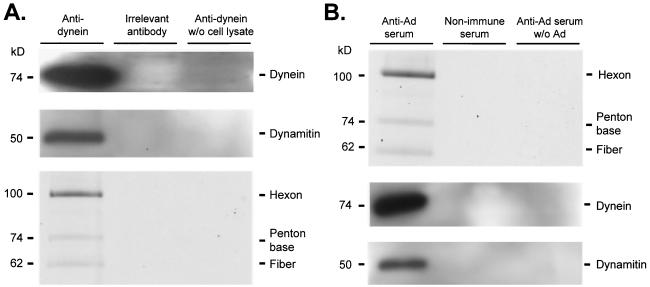
FIG. 6.
Coimmunoprecipitation of cytoplasmic dynein, dynamitin, and Ad. A549 cell lysate was prepared as described in Materials and Methods. Cell lysate in PEM was incubated with either protein A/G or protein L agarose beads for 1 h at 4°C and centrifuged to preclear nonspecific agarose bead binding proteins (three times). Cy3-conjugated Ad serotype 5 capsids (2 × 1010 particles) were added to cell lysate and incubated (40 min at 22°C). Immunoprecipitation antibodies (mouse ascites fluid containing monoclonal anticytoplasmic dynein antibody 74.1, mouse ascites fluid containing anti-horseradish peroxidase antibody, human sera previously characterized to neutralize in vitro Ad infection, and human serum previously demonstrated to be nonneutralizing for in vitro infection by Ad) were incubated for 4 to 8 h at 4°C. Samples containing mouse antibodies were incubated with protein A/G (1 h at 4°C) and centrifuged to isolate antibody and target protein. Samples containing human sera were incubated with protein L beads (1 h, 4°C) and centrifuged to isolate antibody and target protein. Beads were then washed three times with PEM (with 10% glycerol). Immunoprecipitated proteins were analyzed by gel electrophoresis (A) Fluorescence scan and immunoblot of antidynein immunoprecipitated proteins. Cy3-conjugated Ad capsid protein presence was evaluated by a fluorescence scan: hexon (106 kDa), penton base (74 kDa), and fiber (62 kDa) are shown. The presence of dynein (74 kDa) and dynamitin (50 kDa) was evaluated by Western analysis. (B) Immunoblot and fluorescence scan of anti-Ad immunoprecipitated proteins. The presence of dynein (74 kDa) and dynamitin (50 kDa) was evaluated by Western analysis. Cy3-conjugated Ad capsid protein presence was evaluated by a fluorescence scan: hexon (106 kDa) penton base (74 kDa), and fiber (62 kDa) are shown.