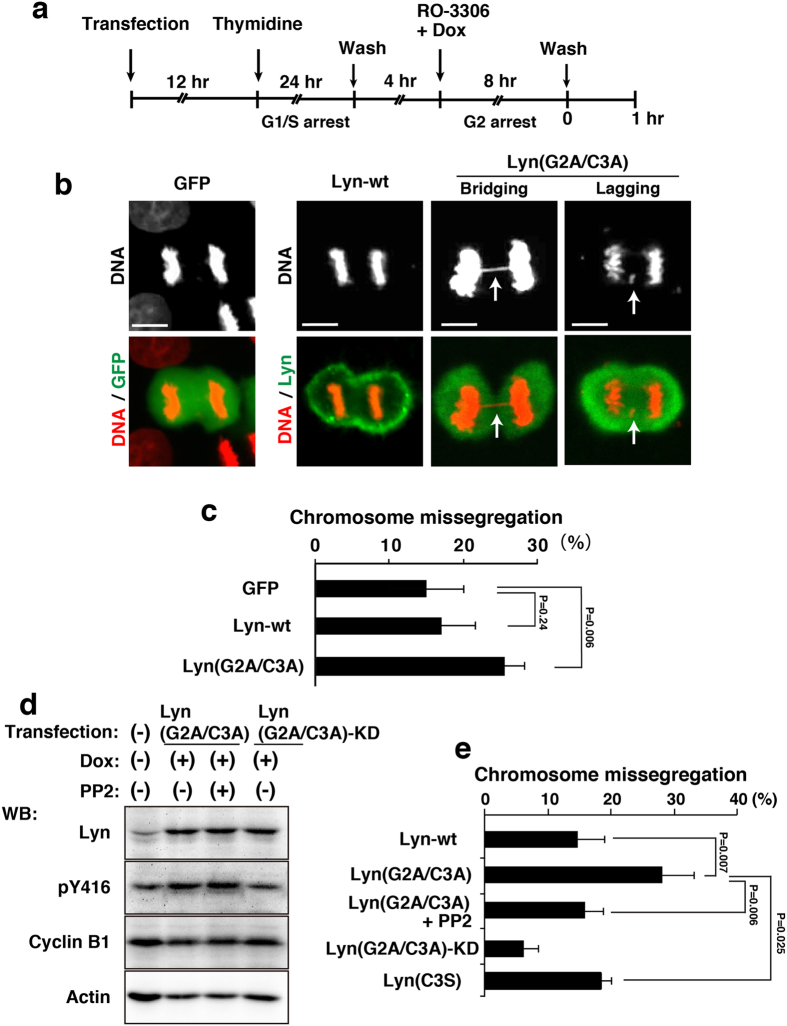
Figure 3. Chromosome missegregation induced by lack of lipid modifications in Lyn.
HeLa S3/TR cells transiently transfected with GFP (control), Lyn-wt, or Lyn(G2A/C3A) were arrested at G1/S phase, released for 4 h, and further treated with 9 μM RO-3306 and 1 μg/ml Dox for 8 h. G2-arrested cells were washed, released into drug-free medium to enter M phase, and fixed after 1 h from the release. (a) Schematic depiction of cell synchronization. (b) Cells entering M phase were stained for GFP and Lyn proteins (green) and DNA (red). Representative images of cells are shown. Scale bars, 10 μm. (c) The percentages of cells exhibiting chromosome missegregation during anaphase and telophase were quantitated (>120 cells). Values are means ± S.D. from more than three independent experiments, and the significant difference is calculated by Student’s t-test. P = 0.24 indicates no significant difference. (d) HeLa S3/TR cells transiently transfected with Lyn(G2A/C3A) or Lyn(G2A/C3A)-KD were synchronized as shown in (a). The cells were treated with 10 μM PP2 for the last 1 h during 8 h-treatment with 9 μM RO-3306 and 1 μg/ml Dox, and then the released cells were further treated with 10 μM PP2 for 1 h. Western blotting was performed with anti-Lyn, anti-Src[pY416] (pY416), anti-cyclin B1, and anti-actin antibodies. A blot with anti-phospho-tyrosine (pTyr) antibody was shown in Supplementary Fig. S2b. Full-length blots are also presented in Supplementary Fig. S2. (e) The cells exhibiting chromosome missegregation (chromosome bridging and lagging) were quantitated (>82 cells). Values are means ± S.D. from more than three independent experiments, and significant differences are calculated by Student’s t-test.