Fig. 4.
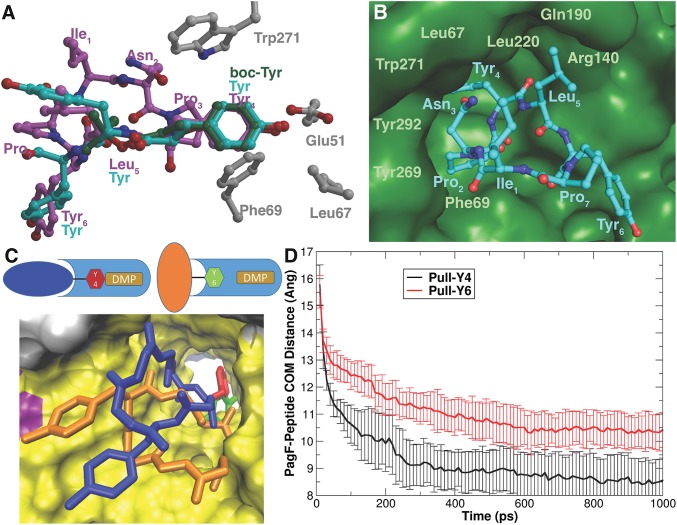
(A) Alignment of linear substrates boc-Tyr (green) and Tyr-Tyr-Tyr (cyan) with the cyclic[INPYLYP] structure (magenta) showing that for all substrates, Tyr is aligned identically among Trp271, Leu67, and Phe69. Glu51 likely serves to deprotonate the phenol to serve in acid/base catalysis. (B) Close-up view of substrate Tyr awaiting isoprene transfer, surrounded by residues that form a hydrophobic chamber. (C) Cartoon illustrating the differences in approach of Tyr4 of cyclic[INPYLYP] vs. Tyr6 to the active site containing DMSPP (DMS; in tan), along with final structures from umbrella sampling. The Tyr4 approach is colored blue/red for cyclic[INPYLYP]/Tyr4 respectively, and the Tyr6 approach is colored orange/green. PagF is colored light blue, and in the structure is shown using a solvent-accessible surface representation looking down the beta barrel toward the active site, colored by secondary structure. Tyr6 cannot penetrate as deeply into the pocket as Tyr4 because of steric interactions of cyclic[INPYLYP] with PagF. (D) Average and SD of the center of mass distance between PagF alpha carbons and cyclic[INPYLYP] alpha carbons during molecular dynamics simulations where either Tyr4 (black line) or Tyr6 (red line) of cyclic[INPYLYP] is guided into the active site of PagF. This figure was generated with Grace 5.1.23.