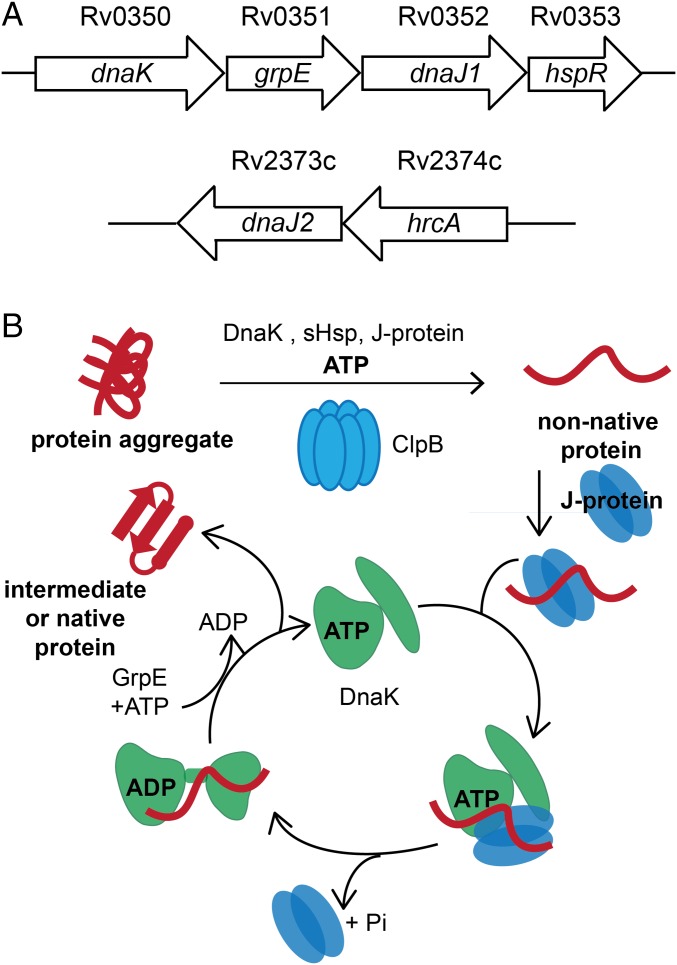
Fig. 1.
The conserved ClpB/DnaK bichaperone network resolves protein aggregates in mycobacteria. (A) Schematic of Mtb dnaK operon containing grpE, dnaJ1 and the regulator hspR. Another gene annotated as dnaJ2 is also present in the Mtb genome with the proposed regulator hrcA. ClpB is encoded by the gene Rv0384c. (B) Schematic of aggregated protein unfolding by the hexamer ClpB and refolding by DnaK with the assistance of the protein cofactors J proteins (DnaJs) and GrpE. DnaK and ClpB are ATPases. J proteins bring aggregated substrates to ClpB/DnaK and enhance ATP hydrolysis by DnaK. GrpE is a nucleotide exchange factor that stimulates release of ADP by DnaK. Small heat shock proteins (sHsps) are proposed to help solubilize aggregates for reactivation. Modified from ref. 8 with permission from the Annual Review of Biochemistry, Volume 82, © by Annual Reviews, www.annualreviews.org.