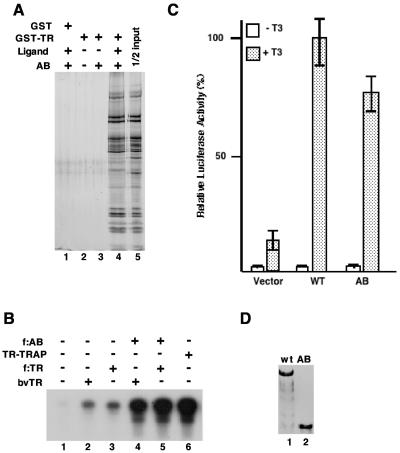
FIG. 4.
The N-terminal AB domain of TRAP220 is sufficient both for interaction with TR and for coactivator function. (A) In the GST pull-down assay, the AB complex (50% of the input is shown in lane 5) was incubated with GST (lane 1) or GST-TRα (lanes 2 to 4) either with no ligand (lanes 2 and 3) or in the presence of 10−7 M T3 (lanes 1 and 4). The retained material was eluted and, following SDS-PAGE, was visualized by silver staining. (B) In vitro transcription assay mixtures were reconstituted from highly purified factors (15), and transcription of a template bearing TR cognate sites (TRE) was monitored. Purified f:AB complex was added to reaction mixtures (lanes 4 and 5). These reaction mixtures also contained TR either from HeLa cells (lanes 3 and 5) or obtained via a baculovirus (bv) expression system (lanes 2 and 4). The TR-TRAP complex (lane 6) was used as a reference. RXRα, but not exogenous ligand, was added to all reaction mixtures. A control template (ML200) that does not contain TR-responsive elements was also included in the reaction mixtures to monitor effects on basal transcription. Although transcription from this template is not apparent at the autoradiography exposure shown, longer exposure revealed no effect of the AB complex on basal transcription. (C) Transient transfection assays were performed with TRAP220−/− MEF cells. Plasmid constructs containing either the full-length TRAP220 (wild type [WT]) or the AB derivative were transfected into the cells in either the presence or absence of T3, as indicated. The empty vector was used as a control. Following transfection, the normalized luciferase activity of the resulting extracts was measured. (D) Immunoblotting to monitor expression of the protein. After TRAP220 and mutant AB proteins were transiently expressed in TRAP220−/− MEF cells, the expressed proteins in nuclei were extracted and probed with anti-HA antibodies.