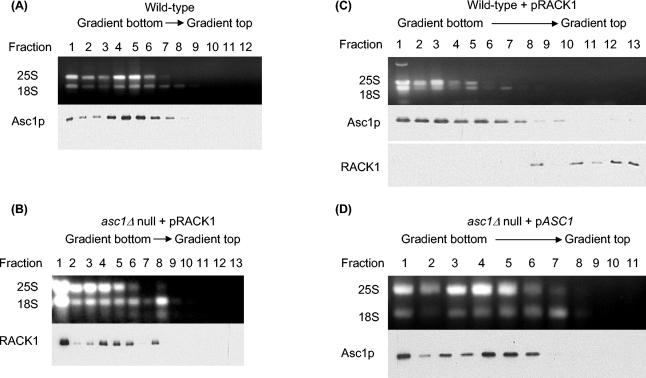
FIG. 1.
Polysome profiles showing that Asc1p and RACK1 are biochemically orthologous ribosomal proteins. (A) Polysome profile showing that yeast Asc1p localizes to the polysome fractions and is absent in the nonribosomal fractions. Cell lysate from yeast wild-type strain AL190 was fractionated by sucrose gradient centrifugation, and fractions were collected. An aliquot of each fraction was analyzed by agarose gel electrophoresis to identify fractions with 25S and 18S rRNAs. Western analysis of the fractions with anti-Asc1p antibodies shows that Asc1p is in the ribosome fractions (lanes 1 to 8) and is absent from the nonribosomal fractions (lanes 9 to 12). (B) Polysome profile showing that RACK1 expressed in a yeast asc1Δ null strain localizes to the ribosomal fractions and is absent from the nonribosomal fractions. Cell lysate from yeast strain AL141 was fractionated by sucrose gradient centrifugation and analyzed as described for panel A except that anti-RACK1 antibody was used for Western analysis. (C) Polysome profile showing exclusion of RACK1 from ribosomal fractions in a wild-type yeast strain. Cell lysate from yeast strain AL140 was fractionated by sucrose gradient centrifugation and analyzed as described for panel A except that anti-RACK1 and anti-Asc1p antibodies were used in separate Western blot assays. (D) Polysome profile of an asc1Δ null strain complemented by expression of ASC1. Cell lysate from yeast strain AL156 was fractionated by sucrose gradient centrifugation and analyzed as described for panel A.