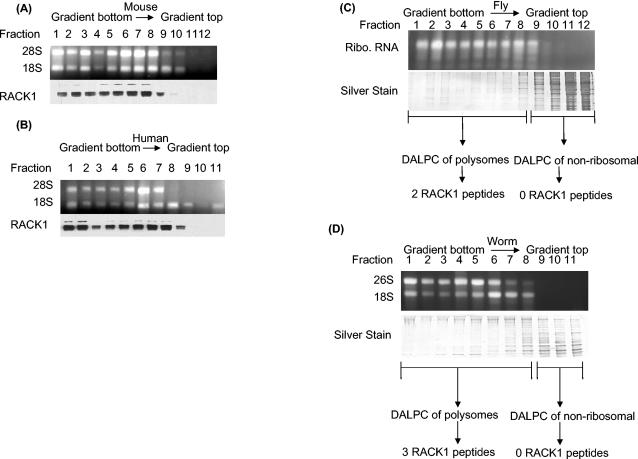
FIG. 2.
Polysome profiling of RACK1 protein showing its polysomal localization in four eukaryotic species. (A) Mouse polysome profile showing that RACK1 localizes to the polysome fractions and is absent from the nonribosomal fractions. A cell lysate from mouse NT2 cells was fractionated by sucrose gradient centrifugation, and fractions were collected. An aliquot of each fraction was analyzed by agarose gel electrophoresis to show fractions with 28S and 18S rRNAs. Western analysis of the fractions with anti-RACK1 antibodies shows the distribution of RACK1. (B) Human polysome profile showing that RACK1 localizes to the polysome fractions and is absent from the nonribosomal fractions. A cell lysate from human HEK293 cells was fractionated by sucrose gradient centrifugation and analyzed as described for panel A. (C) DALPC analysis of the Drosophila polysome profile shows that the putative RACK1 ortholog (NP_477269) localizes to the polysome fractions and is absent from the nonribosomal fractions. Each fraction was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis to visualize the amount of protein in each fraction. Polysome and nonribosomal fractions were separately pooled and digested with trypsin, and proteins were identified by the DALPC-MS approach (34, 47, 63). The Drosophila 28S and 18S rRNAs typically comigrate as a doublet (27). Ribo., ribosomal. (D) DALPC analysis of a nematode polysome profile shows that the putative worm RACK1 ortholog (NP_501859) localizes to the polysome fractions and is absent from the nonribosomal fractions. A lysate from wild-type worms was fractionated by sucrose gradient centrifugation, and fractions were collected. An aliquot of each fraction was analyzed by agarose gel electrophoresis to identify fractions with 28S and 18S rRNAs. Protein samples were analyzed as described for panel C.