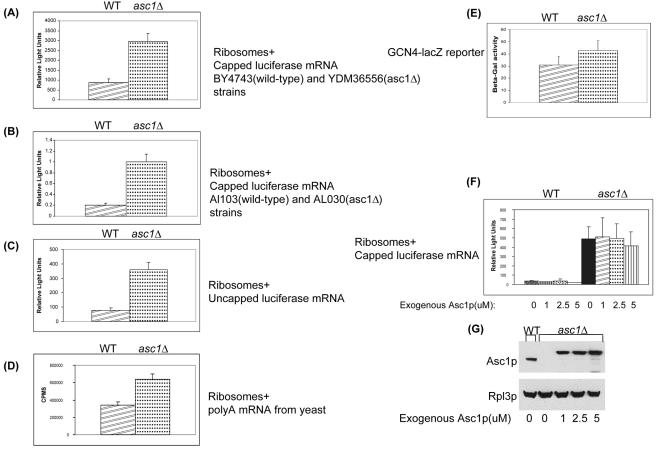
FIG. 4.
Up-regulation of translational activity in asc1Δ extracts. (A) In vitro translation of capped and polyadenylated luciferase reporter mRNA with extracts prepared from wild-type (WT) BY4743 and isogenic asc1Δ null strain YDM36556. Translational activity was determined by measuring luminescence (relative light units) after 30 min of incubation at 26°C. Error bars indicate standard deviations. (B) In vitro translation of capped and polyadenylated luciferase reporter mRNA with extracts prepared from wild-type AL103 and isogenic asc1Δ null strain AL030. (C) In vitro translation of uncapped and polyadenylated luciferase reporter mRNA with extracts prepared from wild-type BY4743 and isogenic asc1Δ null strain YDM36556. (D) In vitro translation of poly(A)-enriched mRNAs with extracts prepared from wild-type BY4743 and isogenic asc1Δ null strain YDM36556. The extracts were incubated with whole wild-type yeast mRNA and [35S]methionine. Translational activity was determined by measuring the counts per min after 30 min of incubation at 26°C. (E) In vivo analysis of GCN4 translation. Wild-type BY4743 and asc1Δ null YDM36556 strains were transformed with a GCN4 5′ UTR-lacZ reporter plasmid (p180) (23). Cells were grown in SC−URA to an OD600 of 0.6 and assayed for β-galactosidase activity as described in Materials and Methods. (F) In vitro translation of capped and polyadenylated luciferase reporter mRNA in the presence of an increasing concentration of recombinant Asc1p protein. Protein extracts were prepared from wild-type BY4743 and isogenic asc1Δ null strain YDM36556. The extracts were incubated with luciferase mRNA and the indicated amounts of recombinant Asc1p protein. Translational activity was determined by measuring luminescence (relative light units) after 30 min of incubation at 26°C. (G) Western analysis of in vitro extracts with anti-Asc1p and anti-Rpl3p antibodies. As a loading control for the in vitro translation assay, Asc1p and Rpl3p levels were measured. Poly-His-tagged recombinant Asc1p migrates more slowly than endogenous Asc1p. Rpl3p levels suggest that 60S subunit levels were similar between wild-type and asc1Δ translation extracts.