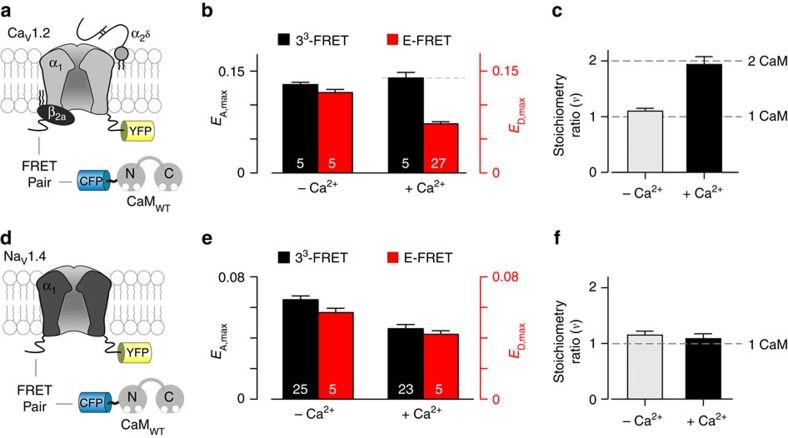
Figure 4. Stoichiometry of calmodulin binding to CaV and NaV channels.
(a) Cartoon illustrates FRET pairs ECFP-CaM and CaV1.2 holochannel tagged with EYFP on its carboxy-terminus. The CaV channel auxiliary subunits β2A and α2δ subunits are coexpressed. (b) Bar-graph summary of maximal 33-FRET (EA,max; black) and E-FRET (ED,max; red) efficiencies under basal (– Ca2+) and elevated Ca2+ conditions for CaM binding to CaV1.2 (mean±s.e.m.; n, number of cells, as labelled for each bar). EA,max and ED,max are approximately equal under resting Ca2+ conditions. With high cytosolic Ca2+ levels, EA,max∼2 × higher than ED,max. (c) Computing stoichiometry ratio (ν) shows that a single CaM binds to the holo-Ca2+ channels under low Ca2+ conditions while two CaM interact with the channel complex upon Ca2+ elevation (mean±s.e.m.). (d) Cartoon depicts FRET pairs ECFP-CaM and NaV1.4 with EYFP fused to its carboxy-terminus. (e) Bar-graph summarizes maximal 33-FRET (EA,max; black) and E-FRET (ED,max; red) efficiencies for CaM binding to NaV1.4 (mean±s.e.m.; n, number of cells, as labelled for each bar). Notice that maximal 33-FRET and E-FRET efficiencies are approximately equal to each when measured under both basal and elevated Ca2+ conditions. (f) Experimentally determined stoichiometry ratio (ν) shows that a single CaM interacts with NaV channel complex under all Ca2+ conditions. Format as in c.