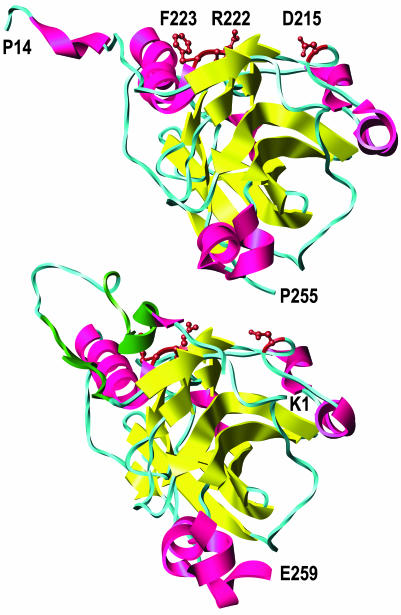
Fig. 7.
Locations of the critical amino acid substitutions (D215, R222, and F223, brown) in gD crystallized alone (Upper) and gD crystallized with HVEM (Lower) (HVEM is not shown). β-Strands are yellow, and α-helices are red except for the contact sites with HVEM (amino acids 7–15 and 24–32), which are dark green. The entire ectodomain of gD is ≈316 aa. A truncated form of gD used for crystallization (amino acids 1–285) yielded structures in which only the first 255 (minus 1–13) or 259 aa were visible. The structures shown are based on the coordinates deposited in the Protein Data Bank (37) for entries 1JMA and 1L2G (19). Molecular graphics images were produced by using the ucsf chimera package (38) from the Computer Graphics Laboratory, University of California, San Francisco (supported by National Institutes of Health Grant P41 RR-01081).