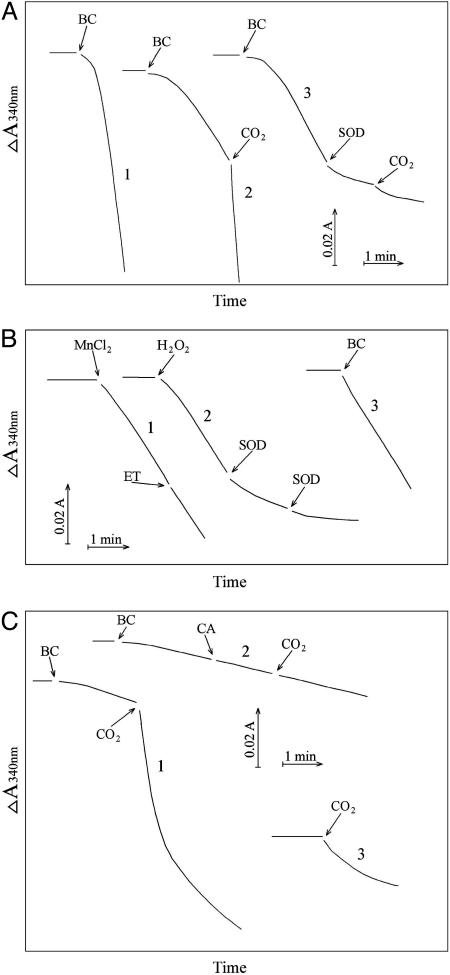
Fig. 7.
Oxidation of NADH. NADH was present at 0.1 mM, and oxidation was followed at 340 nm. (A) Line 1, conditions were as in Fig. 1 except as noted below, and 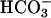 was added to 20 mM at the arrow. Line 2,
was added to 20 mM at the arrow. Line 2, 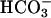 was added to 10 mM at the first arrow, and 0.15 ml of CO2 solution was added at the second arrow. Line 3,
was added to 10 mM at the first arrow, and 0.15 ml of CO2 solution was added at the second arrow. Line 3, 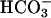 was added to 10 mM at the first arrow, MnSOD was added to 30 μg/ml at the second arrow, and 0.15 ml of CO2 solution was added at the third arrow. (B) Mn(II) was added at 0.1 mM, H2O2 was added at 10 mM, and
was added to 10 mM at the first arrow, MnSOD was added to 30 μg/ml at the second arrow, and 0.15 ml of CO2 solution was added at the third arrow. (B) Mn(II) was added at 0.1 mM, H2O2 was added at 10 mM, and 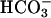 was added at 10 mM. Line 1, Mn(II) was added at the first arrow, and ethanol to 1% was added at the second arrow. Line 2, reaction was started with H2O2 at the first arrow, and then MnSOD was added to 6 μg/ml at the second arrow and to 18 μg/ml at the third arrow. Line 3, as in lines 2 and 3in A but carbonic anhydrase was present at 50 μg/ml. (C) Mn(II) was added at 0.1 mM, H2O2 at 10 mM, and
was added at 10 mM. Line 1, Mn(II) was added at the first arrow, and ethanol to 1% was added at the second arrow. Line 2, reaction was started with H2O2 at the first arrow, and then MnSOD was added to 6 μg/ml at the second arrow and to 18 μg/ml at the third arrow. Line 3, as in lines 2 and 3in A but carbonic anhydrase was present at 50 μg/ml. (C) Mn(II) was added at 0.1 mM, H2O2 at 10 mM, and 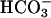 , when present, at 5 mM. Line 1,
, when present, at 5 mM. Line 1, 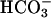 was added at the first arrow, and 0.15 ml of CO2 solution was added at the second arrow. Line 2,
was added at the first arrow, and 0.15 ml of CO2 solution was added at the second arrow. Line 2, 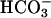 was added at the first arrow, carbonic anhydrase to 50 μg/ml was added at the second arrow, and 0.15 ml of CO2 solution was added at the third arrow. Line 3, 0.15 ml of CO2 solution was added at the arrow, but
was added at the first arrow, carbonic anhydrase to 50 μg/ml was added at the second arrow, and 0.15 ml of CO2 solution was added at the third arrow. Line 3, 0.15 ml of CO2 solution was added at the arrow, but 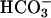 was not present.
was not present.