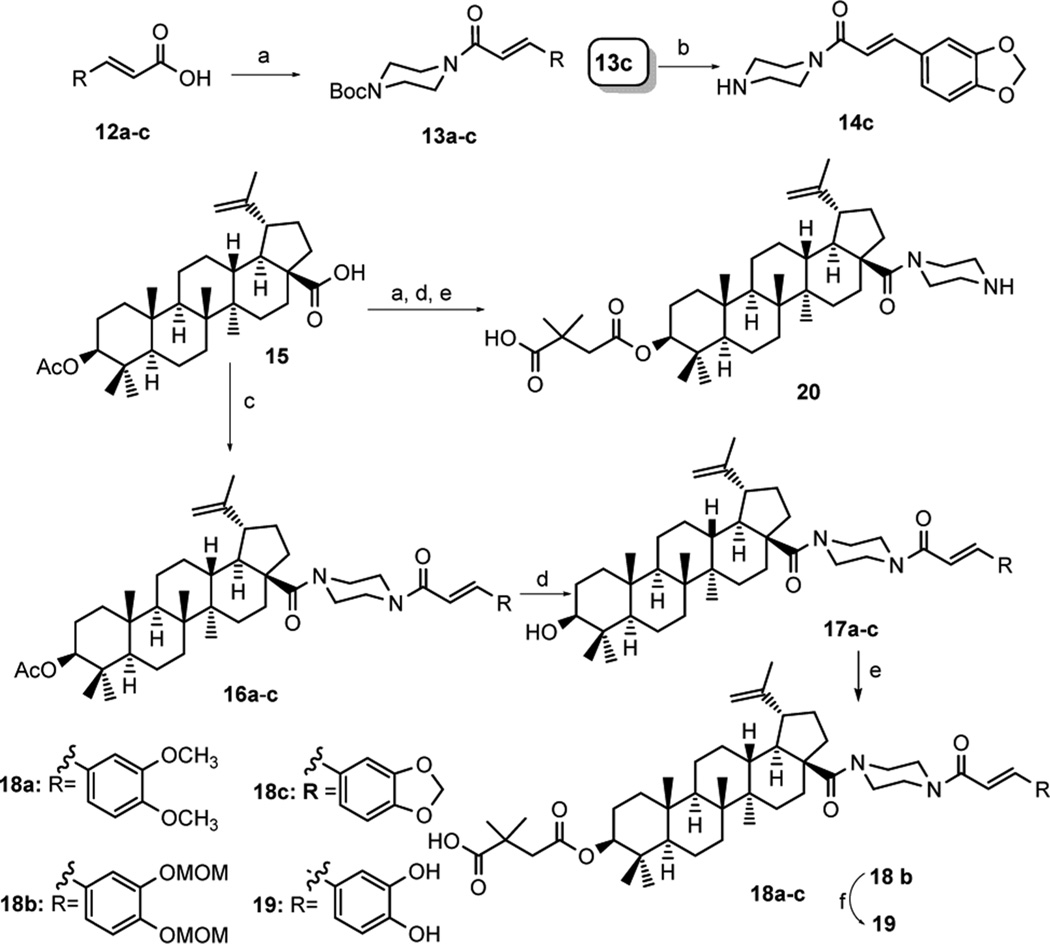
Scheme 1. Synthesis of Compounds 14c, 18a– c, 19, and 20a.
a(a) 1-Boc-piperazine, HOBt, EDCI, Et3N, DCM, overnight; (b) 13a,c TFA, DCM, 2 h; or 13b ZnBr2, DCM, 24 h; (c) oxalyl chloride, DCM, then deprotected 13a–c (for 16a–c) or 1-Boc-piperazine (for 20), Et3N, DCM, 6 h; (d) 4 N NaOH, THF/MeOH, overnight; (e) 2,2-dimethylsuccinic anhydride, DMAP, pyridine, microwave 155 °C, 2 h; (f) 4 N HCl in EtOAc, DCM, 4 h.