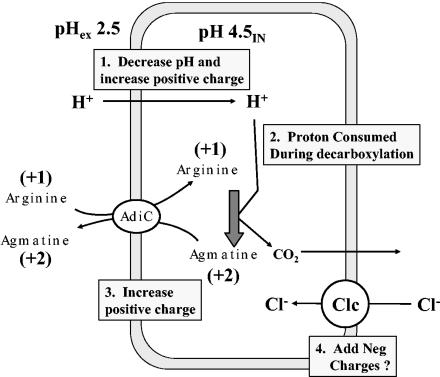
FIG. 6.
Model for amino acid-dependent acid resistance. Acid stress at pH 2.5 results in illicit entry of H+, which decreases pH and increases positive charge. As pHi drops to around 5, arginine decarboxylase will start to consume protons and convert +1-charged arginine to +2-charged agmatine, further increasing the positive charge. An antiporter will not completely drain agmatine from the cell, as it is continually being made during decarboxylation. In this model, the evolution of CO2 does not contribute toward internal pH or charge since (i) the proton donor to make carbonic acid is water, not a proton; (ii) carbonic anhydrase will not function at pH 4.5; and (iii) at this internal pH bicarbonate will not form (pKa = 6.1). The role of the Clc H+:Cl− antiporter is unknown but may help expel H+, limit excess internal positive charge, and aid in returning the cell to an inside negative charge as external pH returns to neutrality.