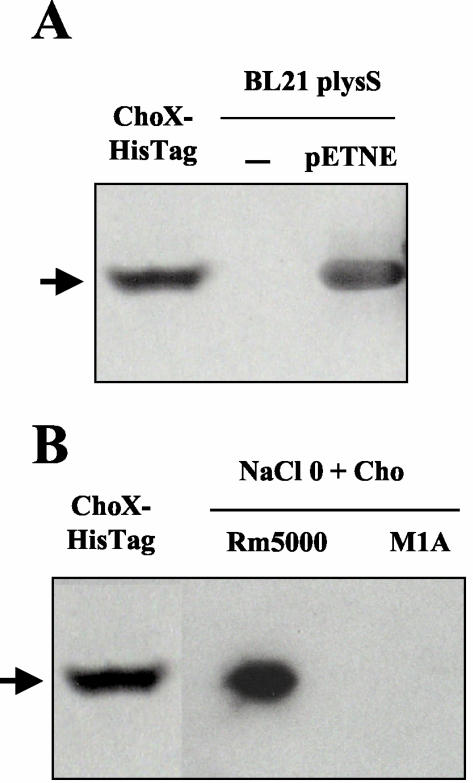
FIG. 3.
Choline-binding activity of ChoX and periplasmic proteins as determined by nondenaturing PAGE. (A) Autoradiography of E. coli proteins from recombinant strain BL21(DE3)(pLysS) expressing ChoX from S. meliloti. Purified His-tagged ChoX (40 μg of protein) and periplasmic proteins (100 μg of protein) from E. coli BL21(DE3)(pLysS) carrying or not carrying the recombinant plasmid pETNE were incubated overnight with [methyl-14C]choline (5.5 kBq; 3.25 nmol), subjected to gel electrophoresis (10% polyacrylamide), and then autoradiographed. E. coli cells were grown in LB medium containing 0.4 mM IPTG and were collected at an optical density at 600 nm of 0.6. (B) Autoradiography of periplasmic proteins from S. meliloti. Cells from wild-type strain Rm5000 and mutant M1A were grown in MCAA medium containing 7 mM choline (Cho) until the stationary phase. Purified ChoX (40 μg) and periplasmic proteins (100 μg) were incubated overnight with [methyl-14C]choline (5.5 kBq; 3.25 nmol) and analyzed as described above. The arrow indicates the position of the ChoX-[14C]choline complex.