Abstract
Several single-domain prokaryotic and eukaryotic cyclophilins have been identified as also being unspecific nucleases with a role in DNA degradation during the lytic processes that accompany bacterial cell death and eukaryotic apoptosis. Evidence is provided here that the supposed nuclease activity of human and bacterial recombinant cyclophilins is due to contamination of the proteins by the host Escherichia coli endonuclease and is not an intrinsic property of these proteins.
Some reports on human recombinant CypA, CypB, and CypC cyclophilins (6), murine CypB cyclophilin (8), Legionella pneumophila LpCyp18 cyclophilin (12), and Streptomyces antibioticus-SanCyp18 cyclophilin (9) describe associated Ca2+/Mg2+ nuclease-dependent activity. For the aforementioned eukaryotic cyclophilins, the accompanying nuclease activity has been proposed as playing a central role in apoptosis. This is the case with NUC-18, a nuclease reported to be identical to rat CypA cyclophilin, involved in glucocorticoid-stimulated apoptosis of thymocytes (7) and murine CypB cyclophilin-associated nuclease, involved in TCR-stimulated apoptosis of thymocytes (8). For S. antibioticus SanCyp18 cyclophilin, nuclease activity has been related to chromosomal DNA degradation during the lysis of substrate (vegetative) mycelium (2), which precedes the emergence of the aerial (reproductive) mycelium (9). Amino acid sequence analysis of the S. antibioticus SanCyp18 protein identified it as a typical cyclophilin that is clearly homologous to cyclophilins from gram-negative bacteria (A. Manteca, T. Kamphausen, J. Fanghanel, G. Fischer, and J. Sanchez, submitted for publication). The biochemical characteristics of SanCyp18 recombinant cyclophilin (binding and inhibition by cyclosporine A and structure of the loop region of the protein) were analogous to those of gram-negative bacteria cyclophilins, from which it may have been passed to Streptomyces species (Manteca et al., submitted).
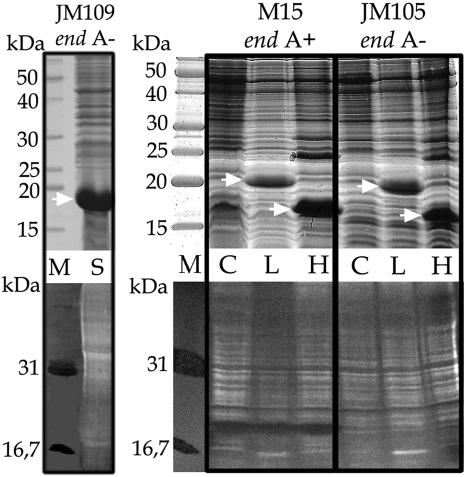
To analyze the hypothetical associated nuclease activity, SanCyp18 cyclophilin was expressed in Escherichia coli JM109(DE3) [endA1 recA1 gyrA96 thi hsdR17 (rK− mK+) relA1 supE44 λ− Δ(lac-proAB) (F′ traD36 proAB lacIqZΔM15) λDE3], a strain lacking periplasmic endonuclease I (14). The nuclease activity of recombinant SanCyp18 was further detected by activity in gel assays, as described formerly (9). The nucleases were separated in a 12% gel containing 10 μg of denatured calf thymus DNA (Sigma)/ml by sodium dodecyl sulfate (SDS) (USB-US75819; Amersham Biosciences)-polyacrylamide gel electrophoresis (PAGE) (9). After electrophoresis, the proteins were renatured by repeatedly washing the gel with renaturation buffer (25 mM Tris-HCl [pH 8.8], 1 mM EDTA, 7 mM β-mercaptoethanol) during 2 h at 4°C. Nuclease activity was visualized by incubating the gels for 1 h at 37°C in 20 mM Tris-HCl (pH 8.0)-7 mM 2-mercaptoethanol-10 mM MgCl2-5 mM CaCl2-10% dimethyl sulfoxide buffer, as reported elsewhere (9), followed by staining with ethidium bromide and analysis under UV light. Micrococcal nuclease and bovine pancreatic DNase I were included as positive controls. As can be seen in Fig. 1, the recombinant protein overexpressed in E. coli JM109(DE3) lacks nuclease activity.
FIG. 1.
Recombinant L. pneumophila LpCyp18, human CypA, and S. antibioticus SanCyp18 cyclophilins were expressed in different E. coli host strains (strain M15, endA+; strains JM109 and JM105, endA mutant). Frozen (−70°C) cells were thawed, resuspended in 15 ml of buffer (20 mM Tris-HCl [pH 8.8], 1 mM EDTA, 7 mM β-mercaptoethanol, 0.5 mM phenylmethylsulfonyl fluoride), and ruptured on ice in an MSE Soniprep 150 ultrasonic disintegrator for 4 cycles of 10 s. After centrifugation at 10,000 rpm in an Eppendorf 5415 R microcentrifuge for 30 min at 4°C, the supernatant was used to analyze protein and nuclease activity. Upper panels, Coomassie-stained proteins (50 μg per lane) analyzed by SDS-PAGE; lower panels, activity gel analysis of the corresponding samples. The strains of E. coli used are indicated at the top of the panels. Lanes M, molecular mass markers (in kilodaltons) (upper panel) and activity gel markers (DNase I, 31 kDa; micrococcal nuclease, 16.7 kDa) (lower panel); lanes C, control E. coli extract without expression vector; lanes L, extract with overexpressed LpCyp18; lanes H, extract with overexpressed human CypA; lane S, extract with overexpressed SanCyp18. Activity is detected as a black band within the brighter background. The white bands along the lanes represent the fragmented chromosomal DNA of E. coli. Arrows show the positions of the recombinant cyclophilins.
The results obtained with S. antibioticus SanCyp18 cyclophilin prompted us to extend the analysis to human CypA and L. pneumophila LpCyp18 cyclophilins, which were also reported to be nucleases by SDS-PAGE activity analysis (see above). We used several E. coli strains as hosts to test the putative nuclease activity for recombinant human CypA (7) and recombinant LpCyp18 (12) cyclophilin. E. coli NM522 and M15 strains were used in the studies mentioned above to express human CypA and LpCyp18, respectively (7, 12). Both of them possess the aforementioned periplasmic endonuclease I, which was absent from the E. coli JM109 strain used to express SanCyp18. This nuclease is detected as a clear band in activity gel assays with E. coli M15 cell extracts (Fig. 1, center panel, lane C). We expressed human CypA and LpCyp18 cyclophilins genes in E. coli M15 (pREP4) (endA+) and E. coli JM105 (pREP4) (endA mutant) (14) by the use of pTTE1 expression vector (12) for the LpCyp18 gene and pTTE2 expression vector (Thomas Tradler, Max-Planck Forschungsstelle “Enzymologie der Proteinfaltung,” Halle/Saale, Germany) for the human CypA gene to investigate the hypothetical associated nuclease activity. The plasmids were introduced in the aforementioned strains to overexpress these proteins, following the protocols described for the QIAGEN system employed. An activity gel assay and SDS-PAGE analysis of the proteins were carried out with the crude E. coli extracts. Although the levels of overexpression were similar in both E. coli strains (Fig. 1), nuclease activity was only observed in the wild-type M15 (endA+) strain. Moreover, the M15 strain extract without any expression vector showed the same nuclease activity level as the M15 strain extracts with the expressed cyclophilins. Finally, the sizes of nuclease and human cyclophilin are different (Fig. 1, center panel, lane H). A previous report showed that, at least for the L. pneumophila LpCyp18 cyclophilin, the hypothetical associated nuclease activity was not linked with its native structure (12). These results are coherent with our finding that the nuclease activity is caused by a different protein. The overall data show that, at least for the recombinant cyclophilins, the E. coli endonuclease is responsible for the activity seen on the gels; therefore, this cannot be attributed to an intrinsic property of cyclophilins. On the other hand, as CsA is a competitive tight-binding inhibitor, it does not inhibit cyclophilin-associated nuclease activity (6). Cyclophilin residues involved in PPIase activity and in CsA binding have been well characterized (3, 5, 10, 11). Both the PPIase domain and the CsA binding sites span the complete length of the amino acid sequence and build a cleft in the core of the three-dimensional structure. As the PPIase active site is efficiently blocked by CsA, an additional nuclease domain would be required to harbor nuclease activity. The small size of most characterized cyclophilins makes such an additional domain very unlikely.
E. coli endonuclease I is a very active periplasmic enzyme whose function is unknown (1, 13). The protein has a putative signal peptide for a cleavage site that would give rise to a mature protein of about 24 kDa (4), consistent with the molecular mass observed in the gel (Fig. 1, center panel, lane C). The 18-kDa Streptomyces nuclease (9) and NUC18 (7) enzymes both exhibit low substrate specificity and high enzymatic activity that can be visualized using SDS-PAGE activity gel assays, even at very low amounts that are not detectable on silver-stained SDS-PAGE (A. Manteca and J. Sanchez, unpublished data). Minor contamination of cyclophilins with these or other nucleases can lead to erroneously associating these activities with the cyclophilin proteins, as occurred in the case of SanCyp18 and LpCyp18 human recombinant cyclophilins. Other native cyclophilins described as nucleases, such as NUC18 (7), murine Cyp B (8), and SanCyp18 (9), might also conceivably be contaminated by host activities. The presence of nuclease-contaminated cyclophilin samples could equally explain some published results on the inhibition of cyclophilin-associated nuclease activity by cyclophilin antibodies (8). In summary, it can be concluded that SanCyp18 and human and Legionella recombinant cyclophilins lack nuclease activity; consequently, other published data concerning the presence of this activity in native cyclophilins should be analogously reappraised.
Acknowledgments
We are grateful to Thomas Tradler, who provided the human CypA clone, and to Paul Barnes for revising the text.
This research was supported by grant BIO2000-0577 from the DGI, Subdirección General de Proyectos de Investigación, MCYT, Madrid, Spain.
REFERENCES
- 1.Durwald, H., H. and Hoffmann-Berling. 1968. Endonuclease-I-deficient and ribonuclease I-deficient Escherichia coli mutants. J. Mol. Biol. 34:331-346. [DOI] [PubMed] [Google Scholar]
- 2.Hodgson, D. A. 1992. Differentiation in Actinomycetes, p. 407-440. In S. Mohan, C. Dow, and J. A. Cole (ed.), Prokaryotic structure and function: a new perspective, vol. 47. Society for General Microbiology Symposium. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- 3.Ivery, M. T. G. 2000. Immunophilins: switched on protein binding domains? Med. Res. Rev. 20:452-484. [DOI] [PubMed] [Google Scholar]
- 4.Jekel, M., and W. Wackernagel. 1995. The periplasmic endonuclease I of Escherichia coli has amino-acid sequence homology to the extracellular DNases of Vibrio cholerae and Aeromonas hydrophila. Gene 154:55-59. [DOI] [PubMed] [Google Scholar]
- 5.Kallen, J., C. Spitzfaden, M. M. G. Zurini, G. Wider, H. Widmer, K. Wülthrich, and D. M. Walkinshaw. 1991. Structure of human cyclophilin and its binding site for cyclosporin A determined by X-ray crystallography and NMR spectroscopy. Nature 353:276-279. [DOI] [PubMed] [Google Scholar]
- 6.Montague, J. W., F. M. Hughes., and J. A. Cidlowski. 1997. Native recombinant cyclophilins A, B and C degrade DNA independently of peptidylprolyl cis-trans-isomerase activity. J. Biol. Chem. 272:6677-6684. [DOI] [PubMed] [Google Scholar]
- 7.Montague, J. W., M. L. Gaido, C. Frye, and J. A. Cidlowski. 1994. A calcium-dependent nuclease from apoptotic rat thymocytes is homologous with cyclophilin. J. Biol. Chem. 269:18877-18880. [PubMed] [Google Scholar]
- 8.Nagata, T., H. Kishi, Q. L. Liu, T. Yoshino, T. Matsuda, Z. X. Jin, K. Murayama, K. Tsukada, and A. Muraguchi. 2000. Possible involvement of cyclophilin B and caspase-activated deoxyribonuclease in the induction of chromosomal DNA degradation in TCR-stimulated thymocytes. J. Immunol. 165:4281-4289. [DOI] [PubMed] [Google Scholar]
- 9.Nicieza, G. R., J. Huergo, B. A. Connolly, and J. Sánchez. 1999. Purification, characterization, and role of nucleases and serine proteases in Streptomyces differentiation. J. Biol. Chem. 274:20366-20375. [DOI] [PubMed] [Google Scholar]
- 10.Plügl, G., J. Kallen, T. Schirmer, N. J. Jansonius, M. M. G. Zurini, and D. M. Walkinshawm. 1993. X-ray structure of a decameric cyclophilin-cyclosporin crystal complex. Nature 361:91-94. [DOI] [PubMed] [Google Scholar]
- 11.Schiene-Fischer, C., J. Habazettl, F. X. Schmid, and G. Fischer. 2002. The hsp70 chaperone DnaK is a secondary amide peptide bond cis-trans isomerase. Nat. Struct. Biol. 9:419-424. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt, B., T. Tradler, J. U. Rahfeld, B. Ludwing, B. Jain, K. Mann, K. P. Rücknagel, B. Janowski, A. Schierhorn, G. Küllertz, J. Hacker, and G. Fischer. 1996. A cyclophilin-like peptidyl-prolyl cis/trans isomerase from Legionella pneumophila-characterization, molecular cloning and overexpression. Mol. Microbiol. 21:1147-1160. [DOI] [PubMed] [Google Scholar]
- 13.Taylor, R. G., D. C. Walker, and R. R. McInnes. 1993. E. coli host strains significantly affect the quality of small scale plasmid DNA preparations used for sequencing. Nucleic. Acids. Res. 21:1677-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]