Table 3.
Evaluation of trisubstituted alkenols
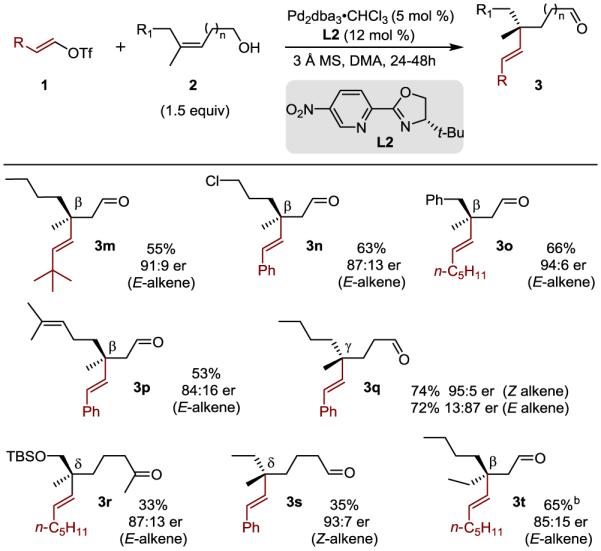
|
Yields are reported as an average of two parallel experiments. Enantioselectivity determined by SFC or GC equipped with a chiral column. Reaction performed on 0.25 mmol scale.
7.5 mol % of Pd2dba3•CHCl3, 16 mol % L2, 2 equivalents of alkenol added and the reaction stirred for 56 hours.
