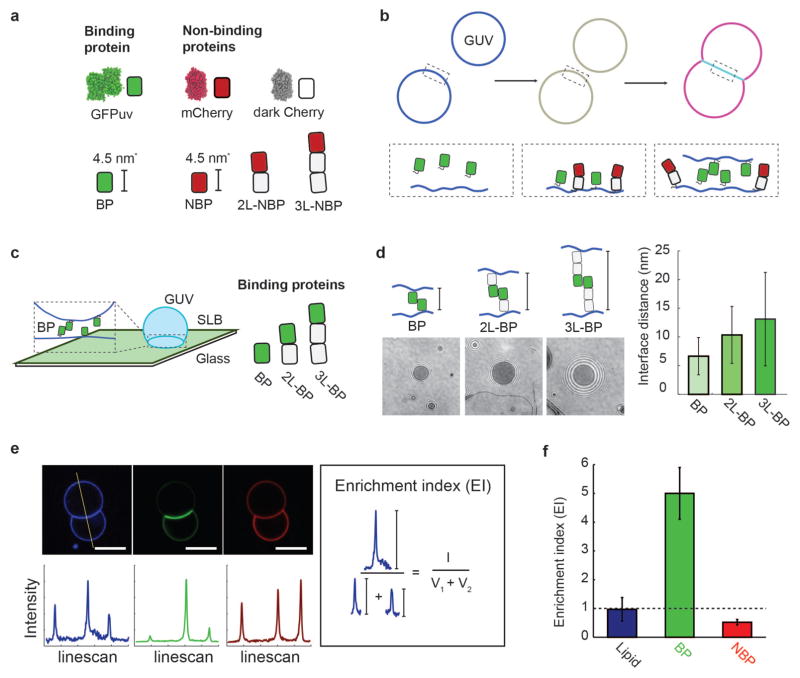
Figure 1.
In vitro membrane interface system. (a) Synthetic binding proteins (GFPuv, BP) and non-binding proteins (mCherry variants, NBP, 2L-NBP, 3L-NBP) are used to investigate protein segregation at membrane interfaces. (b) Membrane interfaces are formed after the synthetic proteins bind to giant unilamellar vesicles (GUVs) that then come into contact. His-tagged binding proteins and non-binding proteins first attach to DOGS-Ni-NTA containing GUV membranes, and then binding protein dimerization leads to interface formation and protein segregation, which can be monitored fluorescently. (c) Membrane interfaces with different gap sizes were formed using binding proteins of different lengths (BP, 2L-BP, 3L-BP) and quantified using reflection interference contrast microscopy (RICM) at an interface formed between a supported lipid bilayer (SLB) and GUV. (d) Representative RICM images at the interface between GUV and SLB for interfaces formed with BP, 2L-BP, and 3L-BP. Image contrast and refractive index differences were used to extract the axial distance between the two membranes. Interface distance increased monotonically with the addition of spacer modules, forming interfaces of 6.2 +/− 3 nm (BP), 10.3 +/− 5.0 nm (2L-BP) or 13.1 +/− 8.1 nm (3L-BP). Error bars are standard deviation across N = 10 vesicles. (e) Representative confocal fluorescence images of GUVs (composition: 97.7 DOPC, 2.5 DOGS-Ni-NTA, 0.3 Atto 390-DOPE) incubated with 100 nM BP and 100 nM 2L-NBP in solution for 10 min. Scale bar is 5 μm long (blue channel: Atto 390-DOPE, green channel: BP, red channel: NBP). Linescans through vesicles dimers allow for quantification of fluorescence intensity at outside vesicle membranes (V1 and V2) and interface (I). We calculate an ‘Enrichment index’ (EI) by taking the ratio between (I) and the sum of (V1 and V2) and reveal uniform distribution of the fluorescently-labeled lipid (EI=1), enrichment of BP (EI>1) and exclusion of 2L-NBP (EI<1) at the interface. Error bars are standard errors of the mean from three independent experiments on separate vesicle batches, each with ~50 vesicles quantified.