Abstract
When investigating the association between brain tumors and use of mobile telephones, accurate data on tumor position are essential, due to the highly localized absorption of energy in the human brain from the radio-frequency fields emitted. We used a point process model to investigate this association using information that included tumor localization data from the INTERPHONE Study (Australia, Canada, Denmark, Finland, France, Germany, Israel, Italy, Japan, New Zealand, Norway, Sweden, and the United Kingdom). Our main analysis included 792 regular mobile phone users diagnosed with a glioma between 2000 and 2004. Similar to earlier results, we found a statistically significant association between the intracranial distribution of gliomas and the self-reported location of the phone. When we accounted for the preferred side of the head not being exclusively used for all mobile phone calls, the results were similar. The association was independent of the cumulative call time and cumulative number of calls. However, our model used reported side of mobile phone use, which is potentially influenced by recall bias. The point process method provides an alternative to previously used epidemiologic research designs when one is including localization in the investigation of brain tumors and mobile phone use.
Keywords: glioma, INTERPHONE Study, intracranial distribution, mobile telephones, radio-frequency electromagnetic fields, spatial point pattern
Use of mobile telephones has increased dramatically within the last 3 decades in most countries (1). The extensive use of mobile phones has been followed by concerns about potential adverse health effects of exposure to radio-frequency electromagnetic fields (RF-EMF) emitted by the devices (2). In a 2011 monograph issued by the International Agency for Research on Cancer, RF-EMF were classified in group 2B, “possibly carcinogenic to humans” (3, 4). The agency's working group considered that the most informative epidemiologic evidence came from the Swedish case-control studies conducted by Hardell et al. (5) and a multinational case-control study, the INTERPHONE Study (6). The latter is the largest investigation of mobile phone use and brain tumors to have been carried out to date. INTERPHONE observed no increased glioma risk in mobile phone users except for the decile with the highest reported cumulative call time (>1,640 hours), with uncertain interpretation (6). National publications on the INTERPHONE data (7–13) and other studies on the association between radio-frequency radiation from mobile phones and brain tumors (14–23) have shown mixed results. When interpreting these findings, the timing of the study, the exposure variables of relevance, and methodological limitations have to be considered (24, 25).
The absorption of energy from RF-EMF in human tissue greatly depends on distance from the source, in addition to factors such as frequency band, network characteristics, and conditions of use (26). Consequently, increased occurrence of tumors in the part of the brain closest to the phone would be expected if there were a causal association. Analyses of all brain tumors together, without localization, are likely to dilute the strength of a risk estimate if a risk is present; hence, it is crucial to include localization. Some studies divided the participants into ipsilateral phone users (phone used on the same side of the head as the tumor) and contralateral phone users (phone used on the opposite side) (6, 9–12, 16, 20–22). Others investigated the risks of brain tumors in the different anatomical lobes of the brain separately (6, 12, 14, 16, 19, 21). Some studies estimated the distance between the brain tumor and the mobile phone and divided cases into those in which the tumor was close to the phone, where most energy from RF-EMF is absorbed, and those in which the tumor was further away (27, 28). Additionally, both the rate of energy absorption (the specific absorption rate (SAR)) inside the tumor (29) and the total cumulative specific energy (TCSE) absorbed for each tumor (30) have been estimated for use as exposure measures.
In the current study, our aim was to use the 3-dimensional point process model of Grell et al. (31) to analyze the INTERPHONE localization data for glioma and thereby further investigate the association between glioma and mobile phone use. Our use of a case-only approach removed possible differential bias between cases and controls, and the specific tumor localization data collected in the INTERPHONE Study allow detailed analysis of intracranial relationships.
METHODS
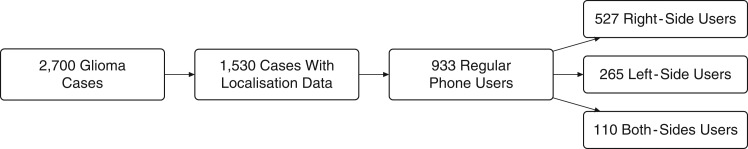
The INTERPHONE Study included participants from 13 countries (Australia, Canada, Denmark, Finland, France, Germany, Israel, Italy, Japan, New Zealand, Norway, Sweden, and the United Kingdom). Cases were between 30 and 59 years of age when diagnosed with a first primary glioma, meningioma, or acoustic neuroma during study periods of 2–4 years between 2000 and 2004 (32). We included only gliomas in our analyses, as their putative origin is less spatially confined in comparison with the origin of meningiomas and acoustic neuromas. The INTERPHONE data comprised 2,700 glioma cases, for which tumor localization was performed by neuroradiologists in 1,530. Localization could not be determined for all cases due to difficulties in retrieving appropriate scans. The computer program GridMaster (Vompras GmbH, Düsseldorf, Germany) was created specifically for recording localizations in the INTERPHONE Study and consisted of a 3-dimensional grid map of the human head and brain made up of 1-cm cubes (voxels). Neuroradiologists recorded the tumor contours and their best estimate of the tumor's point of origin in GridMaster using radiological images (preferably magnetic resonance imaging; otherwise computerized tomography) when available (92.2%) or radiology reports otherwise (7.8%), scaling each brain to match the GridMaster brain. Of the 1,530 tumors with localization data, 906 had a single voxel marked as the putative origin of the tumor, 383 had no origin marked, and 241 had several voxels marked as the origin.
Detailed information on past mobile phone use was collected by interview. The information collected included number of calls, duration of calls, use of a hands-free device, preferred side of the head for mobile phone use, and time since the start of use (approximately 50% of participants were interviewed within 3 months of diagnosis and approximately 90% were interviewed within a year). A regular mobile phone user was defined as a person who had made at least 1 mobile phone call per week for a period of 6 months or more. Among the 1,530 glioma cases with recorded localization data, 933 were regular mobile phone users. The 597 nonregular mobile phone users and nonusers were defined as not being exposed and were not included in our analyses. The lifetime cumulative call time and number of calls, excluding use with hands-free devices, were calculated (32, 33). Overall, levels of use were low compared with today's levels due to the period of data collection, 2000–2004, when mobile phones were less common. Absorbed radio-frequency energy is widely used as a measure of the quantity of radio-frequency exposure in tissue. Calculation of the TCSE was based on an algorithm which included, among other things, self-reported call time, laterality of use, use of hands-free devices, frequency band, communication system, phone class, and network characteristics (34) at each location in the GridMaster brain for the 372 INTERPHONE study subjects with tumor localization data from 5 countries (Australia, Canada, France, Israel, and New Zealand). The INTERPHONE interview included a question about which side of the head mobile phones were generally used on, with “generally” meaning more than 50% of the time. Of the 933 regular mobile phone users, 265 (28.4%) reported using the phone on the left side, 527 (56.5%) reported using it on the right side, and 110 (11.8%) reported both sides; for 31 (3.3%) respondents, the preferred side was unknown.
All diagnoses were histologically confirmed or based on unequivocal diagnostic imaging. From the morphology codes, the tumors were assigned a grade as defined by the World Health Organization (35), but this was only possible for 880 (94.3%) of the regular mobile phone users.
Exposure localization
The ear canals were fully contained within 48 voxels on each side of the GridMaster head, and we defined the location of the exposure source (“the ear”) as the geometric midpoint of the outer area of these voxels. For the GridMaster head, the nearest brain tissue is 15 mm in horizontal distance from the ear, and the midline of the brain is 85 mm in horizontal distance from the ear. We assumed that the energy was emitted at the ear on the side of the head where the mobile phone was reported to generally be used.
Tumor localization
We condensed the tumor localization data for each of the 792 regular mobile phone users with a self-reported preferred side of phone use into a single point. Ideally, this point would represent the origin of the tumor. However, a glioma can grow diffusely and does not necessarily form a single, consolidated mass. Actually, 36 of the 1,530 tumors comprised more than 1 patch of contiguous (sharing either a vertex, edge, or face) voxels. We reviewed a plot of these tumors and decided to include them with all tumor voxels when calculating a tumor's central point. We calculated the tumor localization point as the “center of gravity,” a method which has previously been used in analyses of INTERPHONE data (30). It is the midpoint of the voxel at the shortest distance from the other voxels in the tumor. In the 906 cases with a single voxel marked by the neuroradiologists as the tumor's putative origin, the latter had a mean distance of 4.1 mm from the center of gravity (median, 0 mm; 75th centile, 10 mm; maximum, 51 mm). We also calculated the geometric midpoint of the tumor as an alternative to the center of gravity. The results were similar (see Web Appendix 1, available at http://aje.oxfordjournals.org/).
Statistical analyses
The main point process analysis included all 792 subjects with a self-reported preferred side of use. Each tumor was identified with a single reference location, x = (x1, x2, x3), chosen as the gravity center of the tumor. The ears were identified with locations xL and xR. We assumed the intracranial distribution of tumors in the 2 brain halves to be symmetrical and that the susceptibility of the brain tissue was uniform across each hemisphere.
The point process model is described in further detail elsewhere by Grell et al. (31). Briefly, we assumed that the left-sided users’ and right-sided users’ centers of gravity formed independent Poisson processes with intensities
and
| (1) |
where ρ is a nuisance parameter related to the relative number of left-sided and right-sided users and the baseline intensity λ0(x) reflects the intensity for nonusers. The function g describes the distance relationship between the tumor and the preferred ear. We modeled g as a piecewise constant decreasing function of the distance in millimeters dL = ||x − xL||:
| (2) |
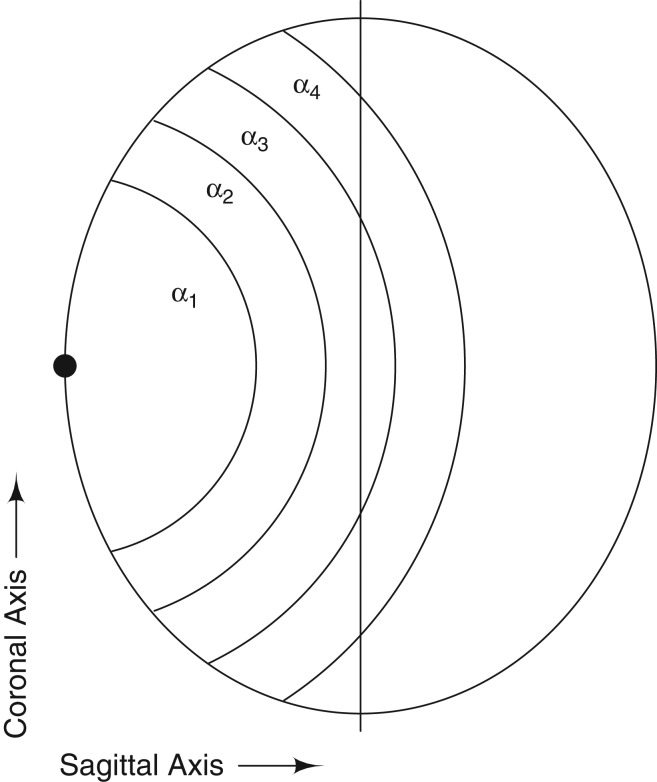
with the added constraint α1 ≥ α2 ≥ α3 ≥ α4 ≥ 1 to ensure a decreasing distance relationship. This was supported by the data subset analyzed by Grell et al. (31). The α values represent the change in risk of observing a tumor within the given interval in comparison with the baseline intensity. We assumed that a possible association with mobile phone use will not affect the contralateral hemisphere; consequently, we fixed g = 1 for distances greater than 115 mm. The null hypothesis (g = 1 or α = 1) is that the occurrence of tumors across each hemisphere for both the left- and right-sided phone users is similar to the occurrence of tumors for persons not using mobile phones. If α is significantly higher than 1, the tumor intensity is significantly higher for the users than the nonusers. Note that the approach does not require the baseline intensity λ0(x) to be estimated (31); hence, the nonusers are not included in the analyses even though they appear in the phrasing of the null hypothesis. Significance testing was done by simulating 1,000 test statistics under the null hypothesis and calculating the empirical P value (31). The reported Monte Carlo confidence intervals are calculated by bootstrapping. The change points in equation 2 were chosen using the actual distances to the preferred ear in the data (39.0–147.7 mm), such that the first 4 intervals were of approximately equal length when taking into account the fact that there was no brain tissue within 15 mm of the ear. Figure 1 is a naive 2-dimensional representation of the GridMaster head and the intervals. The data are from a 3-dimensional model, so α1 covers part of a ball with a radius of 55 mm, α2 a 20-mm layer outside that ball, etc.
Figure 1.
Naive representation of the human head showing intervals from a point process model of the association between brain tumors and mobile telephone use. The radius of α1 is 55 mm, that of α2 is 75 mm, that of α3 is 95 mm, and that of α4 is 115 mm; the short radius of the ellipse is 85 mm.
We dichotomized each of the 7 variables: sex, age, tumor grade, tumor size, time since the start of mobile phone use, lifetime cumulative phone use, and lifetime cumulative number of calls, using the median value for the last 4 variables. If any associations were to be seen in the model, years of phone use and length and number of calls would be related to the exposure; tumor grade and size would be related to the outcome, but they were all entered into the model similarly. We stratified our model for each of these variables z and estimated the 8 parameters corresponding to the model with
| (3) |
We cannot estimate the absolute difference between α0 and α1. Consequently, we cannot assess whether the tumor intensity is higher for one level of the covariate than for the other. However, the model enables us to investigate whether the covariate alters the distance relationship such that the shape of the function g differs between the 2 covariate levels.
The preferred side of the head for phone use did not imply exclusive use at the preferred side; consequently, we redefined our model writing the intensities for left- and right-sided users as mixtures of the distance relationship to the left ear and the right ear:
We chose the mixing proportion wpref = 0.75, which was inspired by the findings of Kiyohara et al. (36).
We conducted several sensitivity analyses. We changed the exposure variable to the distance to the point with the highest SAR instead of the preferred ear. The former is 15 mm in horizontal distance from the latter and coincident with the location of the nearest brain tissue. In this analysis, we redefined the change points in equation 2 by subtracting 15 mm from each of them. Moreover, we changed the exposure variable to the TCSE at the tumor point x, E(x), in a model with
| (4) |
where the change points are the quintiles of TCSE. The interpretation of β is the same as for α: the change in risk of observing a tumor within the given interval compared with the (not estimated) risk in nonusers. We estimated the model with and without the decreasing constraint β1 ≥ β2 ≥ β3 ≥ β4 ≥ 1. These analyses included the 324 cases with preferred laterality out of the total 372 cases with TCSE.
We fitted a model with shorter steps than in equation 2, a model with the mixing proportion specified as wpref = 0.85, and a model with a piecewise constant decreasing-distance relationship for the subsets used in previous case-only analyses: Denmark, Finland, Germany, Italy, Norway, Sweden, and the United Kingdom (n = 428 with preferred laterality out of 515 cases) in the study by Larjavaara et al. (28) and Australia, Canada, France, Israel, and New Zealand (n = 332 with preferred laterality out of 380 cases) in the study by Cardis et al. (30). Because of the uncertainty in the assessment of tumor origin, we conducted the analyses as in the paper by Grell et al. (31), with the same data subset but using the center of gravity to see whether the choice of either point was crucial for these results.
The analyses were carried out using R software, version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria) (37).
RESULTS
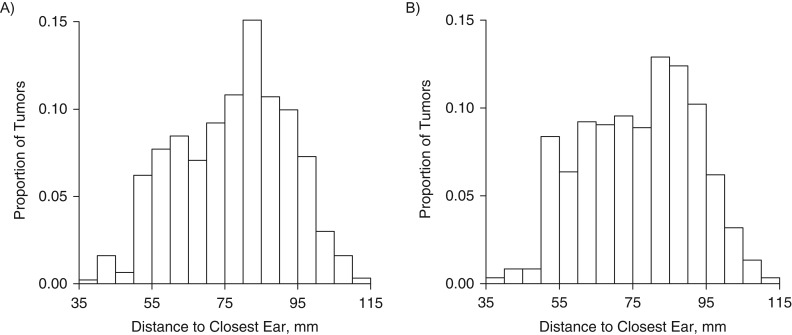
The characteristics of the regular mobile phone users with a self-reported side of use are presented in Table 1, and a flow chart is shown in Figure 2. Figure 3 presents histograms of the distances from the tumor's center of gravity to the closest ear for all regular users and the nonusers; there was no marked difference between the two.
Table 1.
Characteristics of Regular Mobile Telephone Users Who Provided Information on Preferred Side of Usea (n = 792), INTERPHONE Grid Data, 2000–2004
| No. | % | |
|---|---|---|
| Sex | ||
| Male | 508 | 64.1 |
| Female | 284 | 35.9 |
| Age, years | ||
| 30–39 | 224 | 28.3 |
| 40–49 | 257 | 32.4 |
| 50–59 | 311 | 39.3 |
| Tumor grade | ||
| I | 16 | 2.0 |
| II | 315 | 39.8 |
| III | 114 | 14.4 |
| IV | 303 | 38.3 |
| Missing data | 44 | 5.6 |
| Tumor size, no. of voxels | ||
| 1–10 | 240 | 30.3 |
| 11–20 | 201 | 25.4 |
| 21–30 | 138 | 17.4 |
| 31–187 | 213 | 26.9 |
| Time since start of use, years | ||
| 1–3.99 | 273 | 34.5 |
| 4–6.99 | 253 | 31.9 |
| 7–9.99 | 145 | 19.3 |
| 10–22.8 | 121 | 15.3 |
| Cumulative phone use, hours | ||
| 0–29.9 | 207 | 26.1 |
| 30–149.9 | 191 | 24.1 |
| 150–649.9 | 196 | 24.7 |
| 650–211,000 | 198 | 25.0 |
| Cumulative no. of calls | ||
| 0–999 | 235 | 29.7 |
| 1,000–2,999 | 145 | 18.3 |
| 3,000–11,900 | 209 | 26.4 |
| 12,000–506,000 | 203 | 25.6 |
a Side of the head preferred for mobile phone use.
Figure 2.
Numbers of glioma patients included in a study of mobile phone use and preferred side of the head on which the mobile phone was used, INTERPHONE grid data, 2000–2004. Of the 933 regular mobile phone users, 31 had no information on laterality of use.
Figure 3.
Distance between the gravity center of the brain tumor and the closest ear for all regular mobile phone users (n = 933) (A) and all nonusers and nonregular users of mobile phones (n = 597) (B), INTERPHONE grid data, 2000–2004.
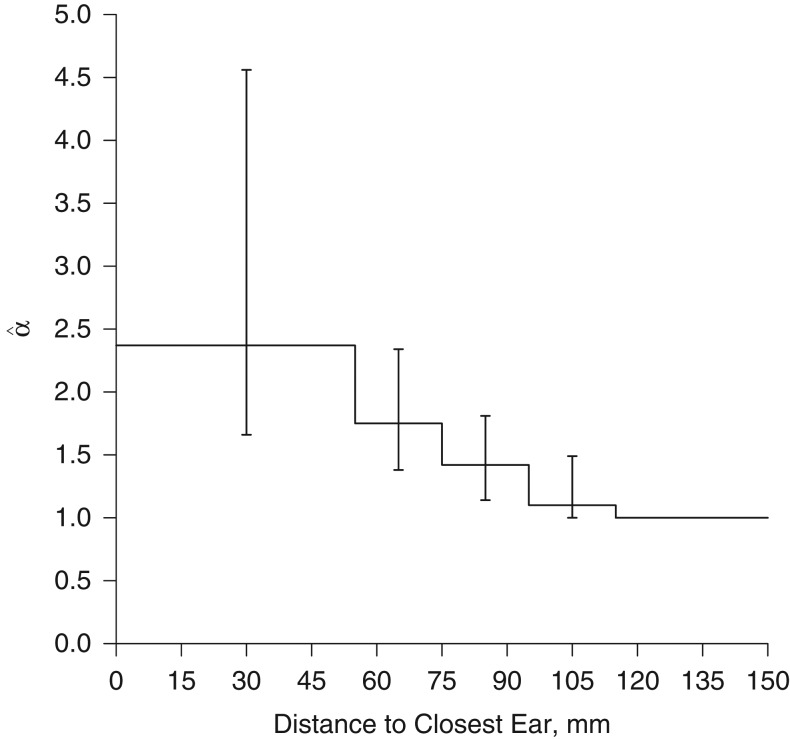
Table 2 shows the estimates and 95% confidence intervals for the model with a piecewise constant decreasing-distance relationship (the "standard" model) (Figure 4), the model with the exposure variable “point with highest SAR,” and the model with mixing proportion wpref = 0.75. The P value for the hypothesis of no association with mobile phone use was less than 0.01 for all 3 models. The estimates for the first 2 models were similar. For the model with the mixing proportion, the estimates were higher but the confidence intervals were also wider.
Table 2.
Estimated Elevation in Brain Tumor Riska for Regular Mobile Phone Users With Information on Preferred Side of Useb (n = 792), INTERPHONE Grid Data, 2000–2004
| Model | Distance From Preferred Ear to Gravity Center of Tumor, mm | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–55 | 55.01–75 | 75.01–95 | 95.01–115 | ≥115.01c | |||||||||||
| No.d | 95% CI | No. | 95% CI | No. | 95% CI | No. | 95% CI | No. | 95% CI | ||||||
| Standard | 45 | 2.37 | 1.66, 4.56 | 159 | 1.75 | 1.38, 2.34 | 220 | 1.42 | 1.14, 1.81 | 166 | 1.10 | 1.00, 1.49 | 202 | 1.00 | N/A |
| Highest SARe | 25 | 2.62 | 1.70, 6.33 | 150 | 1.92 | 1.47, 2.60 | 210 | 1.38 | 1.11, 1.80 | 173 | 1.10 | 1.00, 1.45 | 234 | 1.00 | N/A |
| Mixing wpref = 0.75 f | 45 | 9.66 | 2.84, 39.3 | 159 | 3.50 | 1.96, 8.78 | 220 | 2.09 | 1.36, 3.76 | 166 | 1.28 | 1.00, 2.52 | 202 | 1.00 | N/A |
Abbreviations: CI, confidence interval; N/A, not applicable; SAR, specific absorption rate.
a The values represent the elevation in risk of observing a tumor within a given interval compared with the assumed baseline risk.
b Side of the head preferred for mobile phone use.
c Reference category ( = 1).
d Number of tumors within a given interval.
e The intervals were 0–40 mm, 40.01–60 mm, 60.01–80 mm, 80.01–100 mm, and ≥100.01 mm and measured the distance from the preferred ear to the point with the highest SAR.
f In the model with the mixing proportion, 75% of phone calls were assigned to the preferred side of use and 25% to the nonpreferred side of use.
Figure 4.
Results from a point process model of brain tumor risk with a piecewise constant decreasing-distance relationship for regular mobile phone users with information on preferred side of use, INTERPHONE grid data, 2000–2004. The step function shows values representing the elevation in risk of observing a tumor within a given interval compared with the assumed baseline risk. Vertical bars, 95% confidence intervals.
Table 3 shows results from the piecewise constant decreasing-distance model with the dichotomized covariates included one at a time, including P values from the test of no difference in the distance relationship for the 2 covariate levels. The distance relationship was unrelated to sex, age, tumor grade, tumor size, years of mobile phone use, or amount of mobile phone use, whether measured as cumulative phone use or cumulative number of calls. The test of no association with distance to the mobile phone yielded P < 0.01 for each stratum (not shown).
Table 3.
Estimated Elevation in Brain Tumor Riska for Regular Mobile Phone Users With Information on Preferred Side of Useb (n = 792) From Stratified Models, INTERPHONE Grid Data, 2000–2004
| Covariate | No. of Tumorsc | Distance From Preferred Ear to Gravity Center of Tumor, mm | P Valuee | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–55 | 55.01–75 | 75.01–95 | 95.01–115 | ≥115.01d | ||||||||
| 95% CI | 95% CI | 95% CI | 95% CI | 95% CI | ||||||||
| Sex | 0.26 | |||||||||||
| Female | 284 | 1.85 | 1.41, 4.04 | 1.85 | 1.36, 2.96 | 1.71 | 1.17, 2.44 | 1.00 | 1.00, 1.41 | 1.00 | N/A | |
| Male | 508 | 3.04 | 1.63, 7.54 | 1.68 | 1.26, 2.33 | 1.31 | 1.00, 1.78 | 1.21 | 1.00, 1.64 | 1.00 | N/A | |
| Age, years | 0.39 | |||||||||||
| ≤46 | 379 | 1.86 | 1.45, 4.37 | 1.86 | 1.38, 2.76 | 1.54 | 1.10, 2.09 | 1.00 | 1.00, 1.34 | 1.00 | N/A | |
| >46 | 413 | 3.06 | 1.63, 7.29 | 1.69 | 1.25, 2.51 | 1.40 | 1.03, 1.98 | 1.36 | 1.00, 1.91 | 1.00 | N/A | |
| Tumor gradef | 0.54 | |||||||||||
| 1 or 2 | 331 | 2.59 | 1.45, 6.61 | 1.82 | 1.25, 2.75 | 1.15 | 1.00, 1.76 | 1.15 | 1.00, 1.68 | 1.00 | N/A | |
| 3 or 4 | 417 | 2.16 | 1.46, 5.01 | 1.64 | 1.34, 2.39 | 1.64 | 1.23, 2.13 | 1.08 | 1.00, 1.62 | 1.00 | N/A | |
| Tumor size, cm3 | 0.19 | |||||||||||
| ≤18 | 401 | 1.96 | 1.51, 3.66 | 1.96 | 1.48, 2.97 | 1.70 | 1.21, 2.28 | 1.25 | 1.00, 1.85 | 1.00 | N/A | |
| >18 | 391 | 4.09 | 1.90, 12.0 | 1.51 | 1.17, 2.25 | 1.23 | 1.00, 1.64 | 1.00 | 1.00, 1.40 | 1.00 | N/A | |
| Duration of phone use, years | 0.38 | |||||||||||
| <6 | 461 | 2.02 | 1.31, 4.28 | 1.39 | 1.13, 1.99 | 1.39 | 1.06, 1.81 | 1.00 | 1.00, 1.43 | 1.00 | N/A | |
| ≥6 | 331 | 3.27 | 1.92, 11.3 | 2.32 | 1.57, 3.57 | 1.41 | 1.00, 2.12 | 1.24 | 1.00, 1.85 | 1.00 | N/A | |
| Cumulative phone use, hours | 0.37 | |||||||||||
| <200 | 435 | 1.57 | 1.29, 3.36 | 1.57 | 1.27, 2.22 | 1.48 | 1.10, 1.95 | 1.07 | 1.00, 1.55 | 1.00 | N/A | |
| ≥200 | 357 | 4.06 | 2.03, 11.6 | 1.94 | 1.32, 3.02 | 1.34 | 1.00, 1.97 | 1.13 | 1.00, 1.71 | 1.00 | N/A | |
| Cumulative no. of calls | 0.16 | |||||||||||
| <4,000 | 420 | 1.55 | 1.25, 3.42 | 1.44 | 1.19, 2.02 | 1.44 | 1.10, 1.84 | 1.00 | 1.00, 1.37 | 1.00 | N/A | |
| ≥4,000 | 372 | 3.56 | 2.05, 9.88 | 2.26 | 1.51, 3.38 | 1.39 | 1.03, 2.08 | 1.29 | 1.00, 1.92 | 1.00 | N/A | |
Abbreviations: CI, confidence interval; N/A, not applicable.
a The values represent the elevation in risk of observing a tumor within a given interval compared with the assumed baseline risk.
b Side of the head preferred for mobile phone use.
c Number of tumors within the covariate level.
d Reference category ( = 1).
e Test of no difference in distance relationship between levels of the covariate.
f It was possible to assign tumor grade for only 748 of the 792 regular phone users with information on preferred side of use.
The results from the model including TCSE instead of distance are shown in Table 4 and concur with those for distance, with P < 0.01 when testing g = 1. The association between TCSE and tumor distribution was close to constant after the first interval with the highest TCSE.
Table 4.
Estimated Elevation in Brain Tumor Riska for Regular Mobile Phone Users With Information on Preferred Side of Useb From 5 Countries (Australia, Canada, France, Israel, and New Zealand), Using Total Cumulative Specific Energy Absorbed Instead of Distance From Preferred Ear to Gravity Center of Tumor (n = 324), INTERPHONE Grid Data, 2000–2004
| Model | Total Cumulative Specific Energy,c J/kg | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥3,514.01 | 771.01–3,514 | 186.01–771 | 43.01–186 | 0–43d | |||||||||||
| No.e | 95% CI | No. | 95% CI | No. | 95% CI | No. | 95% CI | No. | 95% CI | ||||||
| Piecewise constant | 82 | 2.38 | 1.33, 5.03 | 57 | 1.03 | 0.58, 1.91 | 58 | 1.02 | 0.57, 1.79 | 66 | 1.10 | 0.66, 1.81 | 61 | 1.00 | N/A |
| Decreasing distancef | 82 | 2.43 | 1.65, 1.57 | 57 | 1.06 | 1.00, 1.96 | 58 | 1.06 | 1.00, 1.70 | 66 | 1.06 | 1.00, 1.64 | 61 | 1.00 | N/A |
Abbreviations: CI, confidence interval; N/A, not applicable.
a The values represent the elevation in risk of observing a tumor within a given interval compared with the assumed baseline risk.
b Side of the head preferred for mobile phone use.
c Values were calculated using the distance from the ear preferred for mobile phone use to the gravity center of the tumor.
d Reference category ( = 1).
e Number of tumors within a given interval.
f Constraint added to the piecewise constant model to ensure decreasing β’s.
Table 5 shows results from the sensitivity analysis comparing the center of gravity with the results from the study by Grell et al. (31) (reported with standard errors as in the paper by Grell et al. (31)). The estimates were similar for both types of tumor points. The results from further sensitivity analyses using the geometric mean, the model with mixing proportion wpref = 0.85, restriction of data to the subsamples from Larjavaara et al. (28) and Cardis et al. (30), and the model with smaller intervals were similar to those presented in Tables 2 and 5 (see Web Appendix 1 (Web Tables 1 and 2) and Web Appendix 2 (Web Tables 3 and 4)).
Table 5.
Comparison of Tumor Points From INTERPHONE Grid Data With the Single-Voxel Tumor Origin Recorded by Neuroradiologists or the Calculated Gravity Center of the Tumor (n = 478), 2000–2004
| Tumor Point | Distance From Preferred Eara to Recorded Origin Point or Gravity Center of Tumor, mm | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–55 | 55.01–75 | 75.01–95 | 95.01–115 | ≥115.01b | |||||||||||
| No.c | d | SE | No. | SE | No. | SE | No. | SE | No. | SE | |||||
| Origin pointe | 25 | 1.82 | 0.32 | 100 | 1.82 | 0.28 | 127 | 1.48 | 0.22 | 105 | 1.09 | 0.18 | 121 | 1.00 | N/A |
| Gravity center | 24 | 1.75 | 0.58 | 105 | 1.68 | 0.24 | 126 | 1.52 | 0.22 | 95 | 1.00 | 0.13 | 128 | 1.00 | N/A |
Abbreviations: N/A, not applicable; SE, standard error.
a Side of the head preferred for mobile phone use.
b Reference category ( = 1).
c Number of tumors within a given interval.
d The values represent the elevation in risk of observing a tumor within a given interval compared with the assumed baseline risk.
e Result from Grell et al. (31).
DISCUSSION
To our knowledge, this was the first analysis to model the intracranial distribution of gliomas in relation to mobile phone use by using the exact localization data from the full INTERPHONE Study. The 3-dimensional distribution of gliomas within the brain was skewed towards the self-reported preferred ear for mobile phone use. This skewness was also found when we considered that the preferred side of the head was not used for all mobile phone calls by assuming that all study participants used the preferred side of the head for 75% of their calls and the nonpreferred side for 25% of the calls. However, we did not find a difference in distance relationship for different levels of lifelong cumulative phone use, and among persons who had used their mobile phone for less than 200 hours, there was still a relationship with distance. Neither did we observe any difference in distance relationship for age, sex, tumor grade, tumor size, years of mobile phone use, or cumulative number of phone calls. We found a significant association between tumor intensity and TCSE, though with lower estimates than for distance alone.
Our results concur with the observation of a statistically significant excess of gliomas on the self-reported side of mobile phone use (28). However, Larjavaara et al. (28) did not observe significantly higher odds for a short distance between glioma and mobile phone for cases than for speculars (a hypothetical control location). Contrary to our method, they considered exposure on the same side of the head as the glioma, irrespective of the reported preferred side of mobile phone use. This avoids potential recall bias but may attenuate any possible association. Our results contrast with the finding in another study of an increase of gliomas for persons with the highest level of TCSE applied only for mobile phone use of more than 7 years (30). Restricting our analysis to the subsets used in the two studies did not markedly change our results.
Studies on the SAR distribution in the human head have shown that energy absorption drops considerably after 5 cm, with almost all energy being absorbed within the brain hemisphere closest to the phone (26). For most of the models, there was a drop after 5.5 cm (between and ); however, this was not as substantial as the drop observed in the studies on SAR. Our data had only a small proportion of tumor points closer than 5 cm to the ear, which could be related to our use of the 3-dimensional gravity point of the glioma. This point has limitations for large, irregularly shaped tumors close to the edge of the brain, because these malignancies may grow towards the center of the brain, resulting in the gravity point being further from the edge and hence further from the exposure. For most of the models, was close to 1, indicating that the size of the association with the phone use was small further than 95 mm away from the phone, in agreement with almost all energy being absorbed within the ipsilateral hemisphere.
The strengths of this study include the large number of cases with localization data and the fact that localization was used as a continuous measure. The use of point process modeling was also a strength; thus, a paired t test comparing distance from the tumor to the preferred ear with distance from the tumor to the opposite ear was insignificant (P = 0.17). Moreover, because our analysis included only cases, the findings were not affected by differential bias between cases and controls (38–41). A limitation of our study is uncertainty about tumors’ points of origin and the fact that the self-reported side of phone use may be influenced by recall bias. Our method necessitated the inclusion of laterality (side of the head) of mobile phone use. Frequently, cases were aware of their tumor location when asked about the preferred side of the head for mobile phone use, which could have caused systematic overreporting of ipsilateral use. In a recent study with healthy volunteers, Kiyohara et al. (36) reported considerable disagreement between self-reported preferred side of mobile phone use (with a recall period of 10–12 months) and that measured by means of a software-modified phone. This indicates that our data on self-reported side of phone use might have been influenced by random recall bias. The proportion of preferred left-sided users versus preferred right-sided users, 0.50 (265/527), was slightly lower than that for the controls from the INTERPHONE Study (0.58 (630/1,082)), who were regular mobile phone users (6). Moreover, the cases reporting a preferred side might not have used the phone exclusively on that side. We dealt with the latter possibility by introducing mixing proportions. This could not eliminate systematic recall bias, but it could ameliorate the parameter estimates by not assuming preferred use to be exclusive use.
Figure 3 shows that distances to the closest ear were similarly distributed for regular users and nonusers, indicating that mobile phone use does not result in tumors being located closer to the ears, overall. Together with the lack of a relationship with phone use, this suggests that our finding could be a result of recall bias.
The main exposure measure in our model was distance between the tumor and the phone, but this is a simplification, because the intracranial distribution of SAR also depends on the frequency band and other characteristics (26, 42). Further, the exposure source was modeled as a single point, though in reality the source is mainly the antenna of the phone, which is frequently embedded in the body of the phone. We modeled the distance relationship as a simple piecewise constant function, and it would have been preferable to also use a model with a continuous distance function, but the data did not support this (31). The model relies on the assumptions that the tumor baseline intensity λ0(x) in the two halves of the brain is symmetrical and is uniform across both hemispheres. This is a simplification, because gliomas occur more frequently in some lobes of the brain than others (43) and the susceptibility of brain tissue is very likely not completely uniform across both hemispheres, because the cells from which gliomas arise are not uniformly distributed in the brain (44).
Taken together, our results suggest that ever using a mobile phone regularly is associated with glioma localization in the sense that more gliomas occurred closer to the ear on the side of the head where the mobile phone was reported to have been used the most. However, this trend was not related to amount of mobile phone use, making it less likely that the association observed is caused by a relationship between mobile phone use and cancer risk. We cannot draw firm conclusions about cause and effect, but our approach has several strengths in comparison with traditional epidemiologic approaches. Our results may have been affected by recall bias in the reported side of phone use. Nevertheless, it provides an alternative for future research related to mobile phone use.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Unit of Statistics, Bioinformatics and Registry, Danish Cancer Society Research Center, Copenhagen, Denmark (Kathrine Grell, Kirsten Frederiksen); Section of Biostatistics, Department of Public Health, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (Kathrine Grell, Per K. Andersen); Section of Environment and Radiation, International Agency for Research on Cancer (IARC), Lyon, France (Joachim Schüz); Centre for Research in Environmental Epidemiology (CREAL), Universitat Pompeu Fabra and Centros de Investigación Biomédica en Red de Epidemiología y Salud Pública (CIBERESP), Barcelona, Spain (Elisabeth Cardis); Sydney School of Public Health, University of Sydney and Sax Institute, Sydney, Australia (Bruce Armstrong); Guzzo-Cancer Research Society Chair in Environment and Cancer, School of Public Health, University of Montreal, Montreal, Quebec, Canada (Jack Siemiatycki); Department of Epidemiology and Community Medicine, Faculty of Medicine, University of Ottawa, Ottawa, Ontario, Canada (Daniel R. Krewski); Cancer Control Research Department, British Columbia Cancer Agency, Vancouver, British Columbia, Canada (Mary L. McBride); Unit of Survivorship, Danish Cancer Society Research Center, Copenhagen, Denmark (Christoffer Johansen); Radiation and Nuclear Safety Authority (STUK), Helsinki, Finland (Anssi Auvinen); Department of Epidemiology, School of Health Sciences, University of Tampere, Tampere, Finland (Anssi Auvinen); Epidemiological Research and Surveillance Unit in Transport, Occupation and Environment, University of Lyon, Lyon, France (Martine Hours); Institute for Medical Biostatistics, Epidemiology and Informatics, University Medical Center, Johannes Gutenberg University Mainz, Mainz, Germany (Maria Blettner); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Ramat Gan, Israel (Siegal Sadetzki); Sackler Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Istituto Superiore di Sanità, National Centre for Epidemiology, Surveillance and Health Promotion, Rome, Italy (Susanna Lagorio); Department of Public Health, School of Medicine, Tokyo Women's Medical University, Tokyo, Japan (Naohito Yamaguchi); Section of Epidemiology and Biostatistics, School of Population Health, University of Auckland, Auckland, New Zealand (Alistair Woodward); Department of Occupational Health Surveillance, National Institute of Occupational Health, Oslo, Norway (Tore Tynes); Institute of Epidemiological Cancer Research, Cancer Registry of Norway, Oslo, Norway (Tore Tynes); Unit of Epidemiology, Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden (Maria Feychting); Division of Epidemiology and Biostatistics, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, United Kingdom (Sarah J. Fleming); Division of Genetics and Epidemiology, Institute of Cancer Research, London, United Kingdom (Anthony J. Swerdlow); and Division of Breast Cancer Research, Institute of Cancer Research, London, United Kingdom (Anthony J. Swerdlow).
This work was supported by funding from the Danish Cancer Society Scientific Committee (grant R20-A897). The INTERPHONE Study was supported by funding from the European Union Fifth Framework Programme (“Quality of Life and Management of Living Resources”; contract QLK4-CT-1999901563) and the International Union Against Cancer (UICC). The UICC received funds for this purpose from the Mobile Manufacturers’ Forum and the Global System for Mobile Communications Association. The Australian study center was supported by the Australian National Health and Medical Research Council (Electromagnetic Energy grant 219129) with funds originally derived from mobile phone service licensing fees; B.A. was supported by a University of Sydney Medical Foundation Program Grant. Cancer Council New South Wales and Cancer Council Victoriaprovided most of the infrastructure for the project in Australia. The Montréal, Québec, Canada, portion of the INTERPHONE Study was primarily funded by a grant from theCanadian Institutes of Health Research (CIHR) (project MOP-42525). Additionally, J.S.’s research team was partly supported by the Canada Research Chairs Program and by the Guzzo Environment-Cancer Research Chair of the Cancer Research Society. The other Canadian study centers were supported by a university-industry partnership grant from the CIHR, the latter including partial support from the Canadian Wireless Telecommunications Association. D.R.K. is the NSERC [Natural Sciences and Engineering Research Council of Canada]/SSHRC [Social Sciences and Humanities Research Council of Canada]/McLaughlin Chair in Population Health Risk Assessment at the University of Ottawa. The Danish study center was supported by the Danish Cancer Society and the Finnish study center by the Emil Aaltonen Foundation and the Academy of Finland. Additional funding for the French portion of the INTERPHONE Study was provided by l'Association pour la Recherche sur le Cancer (contract 5142) and 3 mobile phone network operators (Orange, Société française du radiotéléphone, and Bouygues Télécom). In Germany, additional funds were received from the German Mobile Phone Research Program (Deutsches Mobilfunkforschungsprogramm) of the German Federal Ministry for the Environment, Nuclear Safety, and Nature Protection; the Ministry for the Environment and Traffic of the state of Baden-Württemberg; the Ministry for the Environment of the state of North Rhine-Westphalia; and the MAIFOR Program (Mainzer Forschungsforderungsprogramm) of the University of Mainz. The study conducted in Japan was fully funded by the Ministry of Internal Affairs and Communications of Japan. In New Zealand, funding was provided by the Health Research Council, the Hawkes Bay Medical Research Foundation, the Wellington Medical Research Foundation, the Waikato Medical Research Foundation, and the Cancer Society of New Zealand. The Swedish study center was also supported by the Swedish Research Council and the Swedish Cancer Society. Additional funding for the United Kingdom North and United Kingdom South studies was received from the Mobile Telecommunications, Health and Research Programme, and the United Kingdom North study received funding from the Health and Safety Executive, the Department of Health, the United Kingdom Network Operators (O2, Orange, T-Mobile, Vodafone, “3”), and the Scottish Executive. The Institute of Cancer Research acknowledges National Health Service funding to the National Institute for Health Research Biomedical Research Centre.
We thank Monika Moissonier (IARC) for extracting the data used for this work from the INTERPHONE database and Jordi Figuerola (CREAL) for extracting the total cumulative specific energy data for 5 INTERPHONE countries. We also thank the IARC team that coordinated this study during its fieldwork: Dr. Isabelle Deltour, Dr. Lesley Richardson, Dr. Martine Vrijheid, Monika Moissonnier, Emilie Combalot, and Helen Tardy. Moreover, we thank Dr. Johanna Vompras, who programmed the GridMaster program under the supervision of the German INTERPHONE team and the IARC coordinators, and all of the neuroradiologists who mapped the tumors in GridMaster. We also thank Drs. Graham Giles, Julianne Brown, Marie-Élise Parent, Louise Nadon, Helle Collatz Christensen, Päivi Kurttio, Anna Lahkola, Tiina Salminen, Marlène Bernard, Lucile Montestrucq, Juliet Britton, Gabriele Berg-Beckhoff, Birgitte Schlehofer, Angela Chetrit, Avital Jarus-Hakak, Ivano Iavarone, Toru Takebayashi, Angus Cook, Neil Pearce, Karl G. Blaasaas, Lars Klaeboe, Stefan Lönn, Anders Ahlbom, Patricia A. McKinney, Sarah J. Hepworth, Kenneth R. Muir, Minouk J. Schoemaker, and Juliet Britton for their contributions to the INTERPHONE Study.
Provision of funds to the INTERPHONE Study investigators via the UICC was governed by agreements that guaranteed INTERPHONE's complete scientific independence. The terms of these agreements are publicly available at http://www.iarc.fr/en/researchgroups/RAD/RCAd.html. The CIHR university-industry partnerships program also includes provisions that ensure complete scientific independence of the investigators. The funds provided by the mobile phone network operators in France represented 5% of the total cost of the French study and were governed by contracts guaranteeing the complete scientific independence of the investigators.
Conflict of interest: none declared.
REFERENCES
- 1.International Telecommunications Union Key ICT indicators for developed and developing countries and the world (totals and penetration rates). Geneva, Switzerland: International Telecommunications Union; 2015. http://www.itu.int/en/ITUD/Statistics/Pages/stat/default.aspx. Accessed September 1, 2015. [Google Scholar]
- 2.TNS Opinion & Social Electromagnetic fields. (Special Eurobarometer 347/Wave 73.3). Brussels, Belgium: TNS Opinion & Social; 2010. http://ec.europa.eu/public_opinion/archives/ebs/ebs_347_en.pdf. Accessed September 1, 2015. [Google Scholar]
- 3.International Agency for Research on Cancer. Non-Ionizing Radiation, Part 2: Radiofrequency Electromagnetic Fields. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 102). Lyon, France: International Agency for Research in Cancer; 2013. [PMC free article] [PubMed] [Google Scholar]
- 4.Baan R, Grosse Y, Lauby-Secretan B, et al. . Carcinogenicity of radiofrequency electromagnetic fields. Lancet Oncol. 2011;12(7):624–626. [DOI] [PubMed] [Google Scholar]
- 5.Hardell L, Carlberg M, Mild KH. Pooled analysis of case-control studies on malignant brain tumours and the use of mobile and cordless phones including living and deceased subjects. Int J Oncol. 2011;38(5):1465. [DOI] [PubMed] [Google Scholar]
- 6.The INTERPHONE Study Group. Brain tumour risk in relation to mobile telephone use: results of the INTERPHONE international case-control study. Int J Epidemiol. 2010;39(3):675–694. [DOI] [PubMed] [Google Scholar]
- 7.Christensen HC, Schüz J, Kosteljanetz M, et al. . Cellular telephones and risk for brain tumors: a population-based, incident case-control study. Neurology. 2005;64(7):1189–1195. [DOI] [PubMed] [Google Scholar]
- 8.Schüz J, Böhler E, Berg G, et al. . Cellular phones, cordless phones, and the risks of glioma and meningioma (Interphone Study Group, Germany). Am J Epidemiol. 2006;163(6):512–520. [DOI] [PubMed] [Google Scholar]
- 9.Klaeboe L, Blaasaas KG, Tynes T. Use of mobile phones in Norway and risk of intracranial tumours. Eur J Cancer Prev. 2007;16(2):158–164. [DOI] [PubMed] [Google Scholar]
- 10.Lahkola A, Auvinen A, Raitanen J, et al. . Mobile phone use and risk of glioma in 5 North European countries. Int J Cancer. 2007;120(8):1769–1775. [DOI] [PubMed] [Google Scholar]
- 11.Hepworth SJ, Schoemaker MJ, Muir KR, et al. . Mobile phone use and risk of glioma in adults: case-control study. BMJ. 2006;332(7546):883–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lönn S, Ahlbom A, Hall P, et al. . Long-term mobile phone use and brain tumor risk. Am J Epidemiol. 2005;161(6):526–535. [DOI] [PubMed] [Google Scholar]
- 13.Hours M, Bernard M, Montestrucq L, et al. . Cell phones and risk of brain and acoustic nerve tumours: the French INTERPHONE case-control study [in French]. Rev Epidemiol Sante Publique. 2007;55(5):321–332. [DOI] [PubMed] [Google Scholar]
- 14.Frei P, Poulsen AH, Johansen C, et al. . Use of mobile phones and risk of brain tumours: update of Danish cohort study. BMJ. 2011;343:d6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benson VS, Pirie K, Schüz J, et al. . Mobile phone use and risk of brain neoplasms and other cancers: prospective study. Int J Epidemiol. 2013;42(3):792–802. [DOI] [PubMed] [Google Scholar]
- 16.Inskip PD, Tarone RE, Hatch EE, et al. . Cellular-telephone use and brain tumors. N Engl J Med. 2001;344(2):79–86. [DOI] [PubMed] [Google Scholar]
- 17.Dreyer NA, Loughlin JE, Rothman KJ. Cause-specific mortality in cellular telephone users. JAMA. 1999;282(19):1814–1816. [DOI] [PubMed] [Google Scholar]
- 18.Auvinen A, Hietanen M, Luukkonen R, et al. . Brain tumors and salivary gland cancers among cellular telephone users. Epidemiology. 2002;13(3):356–359. [DOI] [PubMed] [Google Scholar]
- 19.Muscat JE, Malkin MG, Thompson S, et al. . Handheld cellular telephone use and risk of brain cancer. JAMA. 2000;284(23):3001–3007. [DOI] [PubMed] [Google Scholar]
- 20.Hardell L, Carlberg M, Söderqvist F, et al. . Case-control study of the association between malignant brain tumours diagnosed between 2007 and 2009 and mobile and cordless phone use. Int J Oncol. 2013;43(6):1833–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardell L, Carlberg M. Mobile phones, cordless phones and the risk for brain tumours. Int J Oncol. 2009;35:5–17. [DOI] [PubMed] [Google Scholar]
- 22.Hardell L, Carlberg M, Mild KH. Pooled analysis of two case-control studies on use of cellular and cordless telephones and the risk for malignant brain tumours diagnosed in 1997–2003. Int Arch Occup Environ Health. 2006;79(8):630–639. [DOI] [PubMed] [Google Scholar]
- 23.Ali Kahn A, O'Brien DF, Kelly P, et al. . The anatomical distribution of cerebral gliomas in mobile phone users. Irish Med J. 2003;96(8):240–242. [PubMed] [Google Scholar]
- 24.Cardis E, Sadetzki S. Indications of possible brain-tumour risk in mobile-phone studies: should we be concerned. Occup Environ Med. 2011;68(3):169–171. [DOI] [PubMed] [Google Scholar]
- 25.Swerdlow AJ, Feychting M, Green AC, et al. . Mobile phones, brain tumors, and the Interphone Study: where are we now. Environ Health Perspect. 2011;119(11):1534–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardis E, Deltour I, Mann S, et al. . Distribution of RF energy emitted by mobile phones in anatomical structures of the brain. Phys Med Biol. 2008;53(11):2771–2783. [DOI] [PubMed] [Google Scholar]
- 27.Hartikka H, Heinävaara S, Mäntylä R, et al. . Mobile phone use and location of glioma: a case-case analysis. Bioelectromagnetics. 2009;30(3):176–182. [DOI] [PubMed] [Google Scholar]
- 28.Larjavaara S, Schüz J, Swerdlow A, et al. . Location of gliomas in relation to mobile telephone use: a case-case and case-specular analysis. Am J Epidemiol. 2011;174(1):2–11. [DOI] [PubMed] [Google Scholar]
- 29.Takebayashi T, Varsier N, Kikuchi Y, et al. . Mobile phone use, exposure to radiofrequency electromagnetic field, and brain tumour: a case-control study. Br J Cancer. 2008;98(3):652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cardis E, Armstrong BK, Bowman JD, et al. . Risk of brain tumours in relation to estimated RF dose from mobile phones: results from five Interphone countries. Occup Environ Med. 2011;68(9):631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grell K, Diggle PJ, Frederiksen K, et al. . A three-dimensional point process model for the spatial distribution of disease occurrence in relation to an exposure source. Stat Med. 2015;34(23):3170–3180. [DOI] [PubMed] [Google Scholar]
- 32.Cardis E, Richardson L, Deltour I, et al. . The INTERPHONE Study: design, epidemiological methods, and description of the study population. Eur J Epidemiol. 2007;22(9):647–664. [DOI] [PubMed] [Google Scholar]
- 33.Bit-Babik G, Chou CK, Faraone A, et al. . Estimation of the SAR in the human head and body due to radiofrequency radiation exposure from handheld mobile phones with hands-free accessories. Radiat Res. 2003;159(4):550–557. [DOI] [PubMed] [Google Scholar]
- 34.Cardis E, Varsier N, Bowman J, et al. . Estimation of RF energy absorbed in the brain from mobile phones in the Interphone Study. Occup Environ Med. 2011;68(9):686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louis DN, Ohgaki H, Wiestler OD, et al., eds. WHO Classification of Tumours of the Central Nervous System. 4th ed. Lyon, France: International Agency for Research in Cancer; 2007. [Google Scholar]
- 36.Kiyohara K, Wake K, Watanabe S, et al. . Recall accuracy of mobile phone calls among Japanese young people [published online ahead of print March 18, 2015]. J Expo Sci Environ Epidemiol. (doi: 10.1038/jes.2015.13). [DOI] [PubMed] [Google Scholar]
- 37.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 38.Vrijheid M, Deltour I, Krewski D, et al. . The effects of recall errors and of selection bias in epidemiologic studies of mobile phone use and cancer risk. J Expo Sci Environ Epidemiol. 2006;16(4):371–384. [DOI] [PubMed] [Google Scholar]
- 39.Vrijheid M, Cardis E, Armstrong BK, et al. . Validation of short term recall of mobile phone use for the Interphone Study. Occup Environ Med. 2006;63(4):237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vrijheid M, Armstrong BK, Bèdard D, et al. . Recall bias in the assessment of exposure to mobile phones. J Expo Sci Environ Epidemiol. 2009;19(4):369–381. [DOI] [PubMed] [Google Scholar]
- 41.Vrijheid M, Richardson L, Armstrong BK, et al. . Quantifying the impact of selection bias caused by nonparticipation in a case-control study of mobile phone use. Ann Epidemiol. 2009;19(1):33–41. [DOI] [PubMed] [Google Scholar]
- 42.Deltour I, Wiart J, Taki M, et al. . Analysis of three-dimensional SAR distributions emitted by mobile phones in an epidemiological perspective. Bioelectromagnetics. 2011;32(8):634–643. [DOI] [PubMed] [Google Scholar]
- 43.Larjavaara S, Mäntylä R, Salminen T, et al. . Incidence of gliomas by anatomic location. Neuro Oncol. 2007;9(3):319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker C, Baborie A, Crooks D, et al. . Biology, genetics and imaging of glial cell tumours. Br J Radiol. 2011;84(spec no. 2):S90–S106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.