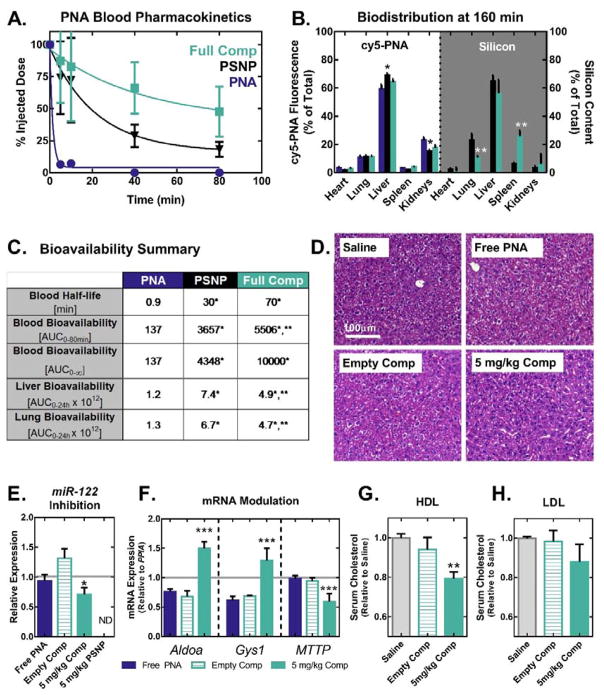
Figure 3.
PSNP-polymer nanocomposites increase PNA blood circulation half-life, bioavailability, and anti-miRNA activity in vivo. (A) Blood pharmacokinetics curves generated using cy5-labeled PNA show that PSNPs increase circulation half-life of PNA when delivered I.V. through the tail vein of mice at a 1 mg kg−1 dose (n=8 per group for 0–20 min, n=5 per group for 40–80 min). (B) In vivo biodistribution of cy5-PNA cargo and Si from the PSNP carriers was analyzed by fluorescent imaging and ICP-OES, respectively. PNA and Si organ distributions 160 min after injection show that PSNPs increase PNA accumulation in the liver and decrease uptake in the kidneys. (C) Quantification of bioavailability in blood, liver, and lungs demonstrates that PEGDB functionalization improves blood circulation stability and decreases particle lung accumulation. (D–H) In vivo miR-122 inhibition studies following injection of a 5 mg kg-1 dose of PNA, every other day for 6 days (n=6 mice per group). (D) On day 6, livers were formalin fixed and paraffin embedded, and stained with H&E. Livers were then evaluated by an experienced veterinary pathologist blinded to the composition of the groups, who found no evidence of liver toxicity observed microscopically (representative image shown, n=6 mice per group). (E) PCR of RNA extracted from livers on day 6 reveals that nanocomposite delivery of anti-miR122 PNA (D) significantly inhibits miR-122 and (F) modulates the expression of miR-122 direct target genes, Aldoa and Gys1, in addition to the indirect target gene MTTP. (Grey line indicates saline control). Cholesterol measurements on (G) high-density lipoprotein (HDL) and (H) low-density lipoprotein (LDL) fractions separated by FPLC from plasma collected on day 6 reveals decreased cholesterol in HDL following treatment with nanocomposites loaded with anti-miR-122 PNA. (*, **, and *** indicate p<0.05 when compared to free PNA, PSNPs, and empty vehicle control, respectively).