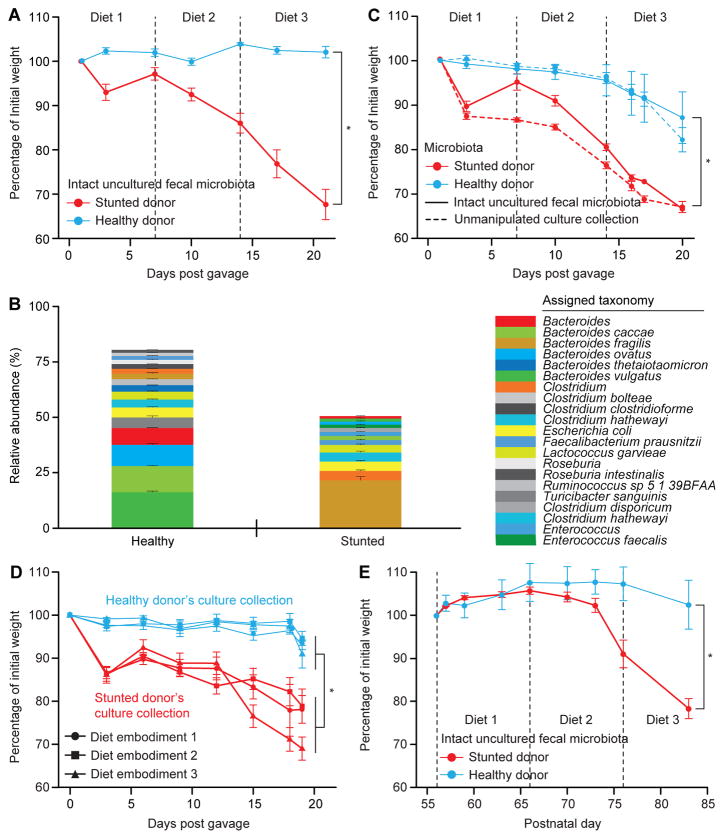
Fig. 1. Intact uncultured human fecal microbiota and derived culture collections from healthy and undernourished Bangladeshi children transmit discordant weight phenotypes to gnotobiotic mice.
(A) 8–9 week old germ-free male C57BL/6J mice (n=8/treatment group) gavaged with intact uncultured fecal microbiota from Bangladeshi donors were fed a sequence of three embodiments of a representative Bangladeshi diet consumed by members of the donor population. See Fig. S1A for experimental design. Mean weights (± s.e.m.) as a function of days post gavage (dpg) are shown as percentages of weights immediately prior to fecal microbiota transplantation. (B) Efficiency of capture of bacterial OTUs present in the donor’s intact uncultured fecal samples in gnotobiotic mice. Mean relative abundances (± s.e.m.) of 97%ID OTUs representing ≥1% of the total fecal microbial communities in recipient animals. Results are based on V4-16S rRNA datasets and summarized at the species level (or genus when species could not be determined). OTUs present at lower abundances are not shown and account for the proportion not represented in each stacked barplot. (C) Transplantation of culture collections (dashed lines) generated from the fecal microbiota of the healthy or stunted/underweight donors recapitulated the discordant weight phenotype seen with the corresponding intact uncultured microbiota (solid lines). (n=6 mice/treatment group, mean weights ± s.e.m. plotted). *, p<0.05 (paired two-tailed Student’s t-test and linear mixed effects model as above). (D) The weight-loss phenotype observed in recipients of the stunted/underweight donor’s culture collection is not significantly different between the three Bangladeshi diet embodiments tested (p>0.05; two-tailed Student’s t-test). Mean weights (± s.e.m.) are plotted as a function of dpg (n=6 mice/culture collection/diet embodiment). Significant weight differences were seen between mice colonized with the healthy donor’s compared to the stunted/underweight donor’s culture collection in the context of all three embodiments of the Bangladeshi diet. *, p<0.05 (paired two-tailed Student’s t-test and linear mixed effects model as above). (E) Intergenerational transmission of discordant weight phenotypes. See Fig. S1C for experimental design. Mean weights (± s.e.m.) of offspring of female gnotobiotic mice colonized with the indicated donors’ microbiota (n=3–4 mice/treatment group) are plotted as a function of age. Animals were switched from a nutrient sufficient low fat, high plant polysaccharide mouse chow to embodiments of the Bangladeshi diets beginning on postnatal day 56. *, p<0.05 (tested by both paired two-tailed Student’s t-test comparing weights at sacrifice, and linear mixed effects model assessing interaction of weight, dpg, and microbiota through the experiment). The efficiency of intergenerational transmission of 97% ID OTUs was 96±1.8% and 88±2.3% (mean ± s.e.m.) for the healthy and stunted/underweight donor’s microbiota, respectively (defined at the time of euthanasia).