Abstract
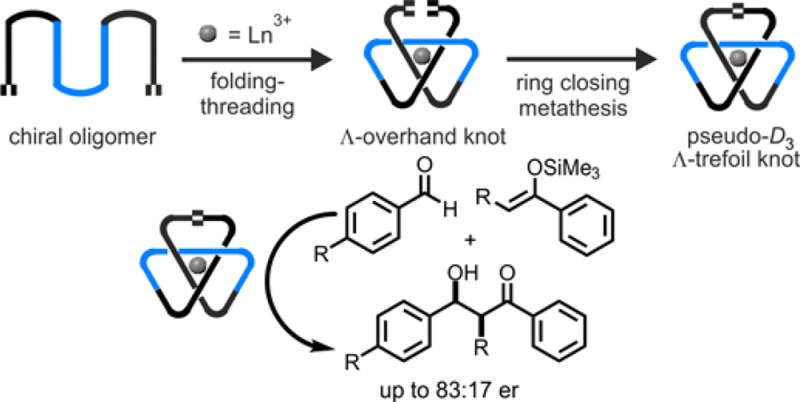
We report the stereoselective synthesis of a left-handed trefoil knot from a tris(2,6-pyridinedicarboxamide) oligomer with six chiral centers using a lanthanide(III) ion template. The oligomer folds around the lanthanide ion to form an overhand knot complex of single handedness. Subsequent joining of the overhand knot end groups by ring-closing olefin metathesis affords a single enantiomer of the trefoil knot in 90% yield. The knot topology and handedness were confirmed by NMR spectroscopy, mass spectrometry, and X-ray crystallography. The pseudo-D3-symmetric knot was employed as an asymmetric catalyst in Mukaiyama aldol reactions, generating enantioselectivities of up to 83:17 er, which are significantly higher than those obtained with a comparable unknotted ligand complex.
Knotted regions of proteins can play a significant role in ligand binding1 and alter enzymatic activity compared with unknotted homologues.2 The chemical effects of knotting in synthetic molecular systems, however, have been less explored.3 The simplest nontrivial knot, the trefoil knot, has three crossings and is topologically chiral.4 Although a number of synthetic strategies to racemic trefoil knots have been developed,5,6 there are few examples of their stereoselective synthesis.7 Here we describe the assembly of a trefoil knot of single handedness by entwining a ligand strand with six asymmetric carbon atoms around a lanthanide ion template.8 We find that the chiral trefoil knot is an effective catalyst for the asymmetric Mukaiyama aldol reaction. As far as we are aware, this is the first example of a chiral molecular knot being utilized in asymmetric catalysis.9,10
We recently described7d the assembly of three chiral 2,6-pyridinedicarboxamide ligands about a lanthanide metal ion to form a circular helicate.11 Joining the ligands’ end groups afforded a trefoil knot of single handedness, with the point chirality of the ligands determining the topological handedness of the knot. Following Hunter’s synthesis of a racemic trefoil knot by entwining a flexible bipyridine oligomer about a zinc(II) ion template to form a racemic overhand knot,5g,12 we envisioned that it might be possible to tie an overhand knot of defined stereochemistry in a molecular strand using a chiral tris(2,6-pyridinedicarboxamide) oligomer and a lanthanide(III) ion template. Subsequently joining the ends of the overhand knot together would give a chiral trefoil knot.
Our previous Ln-template chiral trefoil knot was prepared7d using ring-closing olefin metathesis (RCM) to simultaneously form 10-atom linkers between each pair of the three 2,6-pyridinedicarboxamide units. To maintain a similar spacer length13 in the tris(2,6-pyridinedicarboxamide) oligomer, we used triethylene glycol groups to connect the end sections to the central ligand set, generating ligand (R6)-1 (see the Supporting Information).
Ligand (R6)-1 was treated with Lu(CF3SO3)3 in CD3CN, and the overhand knot tying process (Scheme 1, step a) was followed by 1H NMR spectroscopy (Figure 1). Although the assembly of discrete 2,6-pyridinedicarboxamide ligands about a lanthanide(III) ion is typically fast, even at room temperature,7d,14 the 1H NMR spectrum of (R6)-1 in the presence of Lu(CF3SO3)3 was initially broad (Figure 1b). However, heating the solution at 80 °C led to a sharp 1H NMR spectrum after several hours (Figure 1c), indicating slow equilibration to predominantly a single species. This was shown to be the overhand knot complex Λ-Lu(R6)-1(CF3SO3)3 by a combination of electrospray ionization mass spectrometry (ESI-MS) (m/z 1074 [Lu(R6)-1][CF3SO3]2+, 666 [Lu(R6)-1]3+) and 1H NMR spectroscopy (Figure 1c).
Scheme 1. Synthesis of Molecular Overhand Knots Λ-Lu/Eu(R6)-1(CF3SO3)3 and Trefoil Knots Λ-Lu/Eu(R6)-2(CF3SO3)3 of Single Handedness.
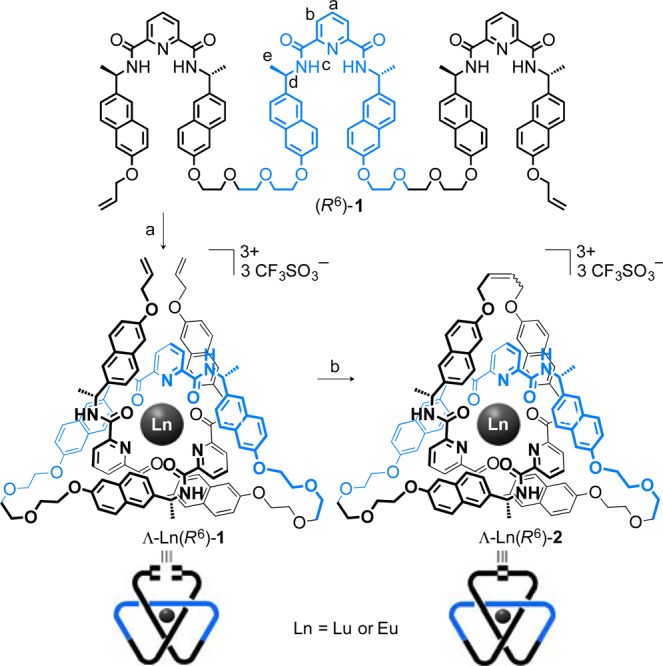
Reagents and conditions: (a) Eu(CF3SO3)3, CH3CN, 80 °C, 12 h, 85% or Lu(CF3SO3)3, 80 °C, 12 h, 90%; (b) Hoveyda–Grubbs second-generation catalyst (15 mol %), CH2Cl2/CH3NO2, 50 °C, 18 h, 88% Λ-Lu(R6)-2(CF3SO3)3, 90% Λ-Eu(R6)-2(CF3SO3)3.
Figure 1.
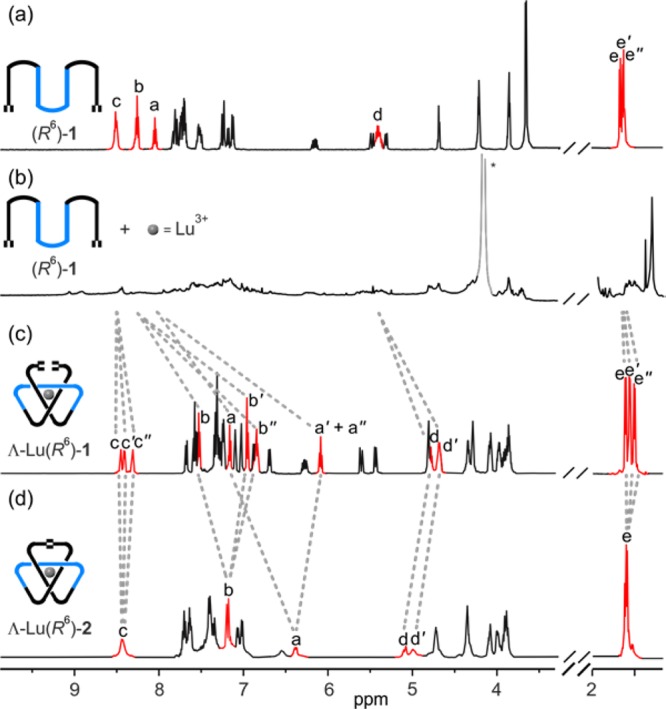
Selected regions of the 1H NMR spectra (600 MHz, CD3CN, 295 K [345 K for (a) and (c)]) of (a) ligand strand (R6)-1, (b) an equimolar mixture of oligomer (R6)-1 and Lu(CF3SO3)3 after 5 min at rt, (c) the left-handed overhand knot complex Λ-Lu(R6)-1(CF3SO3)3, (d) and the left-handed trefoil knot complex Λ-Lu(R6)-2(CF3SO3)3. The signals shown in red correspond to the protons in the pyridine rings and −NHCHCH3– fragments. The lettering refers to the proton assignments shown in Scheme 1. * = water.
The 1H NMR spectrum of Λ-Lu(R6)-1(CF3SO3)3 (Figure 1c) features several different environments for each set of protons Ha, Hb, Hc, Hd, and He, consistent with the pronounced asymmetric environment provided by the overhand knot. The pyridine ring protons Ha and Hb are significantly shifted upfield with respect to those in (R6)-1 (ΔδHa = 0.89 and 1.96 ppm), indicative of π–π stacking between the pyridine and naphthalene rings. The Hd protons are also upfield shifted (Δδ = 0.63 and 0.73 ppm) and split into two different signals.
To join the two end groups of the overhand knot, Λ-Lu(R6)-1(CF3SO3)3 was treated with the second-generation Hoveyda–Grubbs catalyst in 3:1 (v/v) CH2Cl2/CH3NO2 at 50 °C for 18 h (Scheme 1, step b). Quenching of the reaction with ethyl vinyl ether followed by addition of dichloromethane precipitated the trefoil knot complex Λ-Lu(R6)-2(CF3SO3)3. The 1H NMR spectrum (Figure 1d) lacks the terminal alkene protons of Λ-Lu(R6)-1(CF3SO3)3 (Figure 1c) and features fewer sets of resonances than the overhand knot, a reflection of the trefoil knot being essentially D3-symmetric except that one of the three linker groups is different from the other two. ESI-MS confirmed the intramolecular ring closure (m/z 1060 [Lu(R6)-2][CF3SO3]2+, 657 [Lu(R6)-2]3+).
Substituting Eu(CF3SO3)3 for Lu(CF3SO3)3 in the reactions shown in Scheme 1 generated the corresponding europium trefoil knot complex Λ-Eu(R6)-2(CF3SO3)3 (see the Supporting Information). Slow diffusion of diethyl ether into a saturated methanolic solution of Λ-Eu(R6)-2(CF3SO3)3 afforded single crystals suitable for X-ray diffraction. The solid-state X-ray structure confirmed the molecular topology and showed that the trefoil knot is of Λ handedness (Figure 2 and the Supporting Information). The knotted ligand wraps around the europium ion to give a trigonal-prismatic coordination geometry with the Eu–O (2.33 and 2.39 Å) and Eu–N (2.52 Å) distances in the expected ranges for europium-2,6-pyridinedicarboxamide complexes.7d,15 Aromatic stacking between each pyridine ring and two naphthalene groups holds the ligand in a compact arrangement around the metal ion. The solid-state structure is consistent with the 1H NMR shielding observed in solution (Figure 1).
Figure 2.
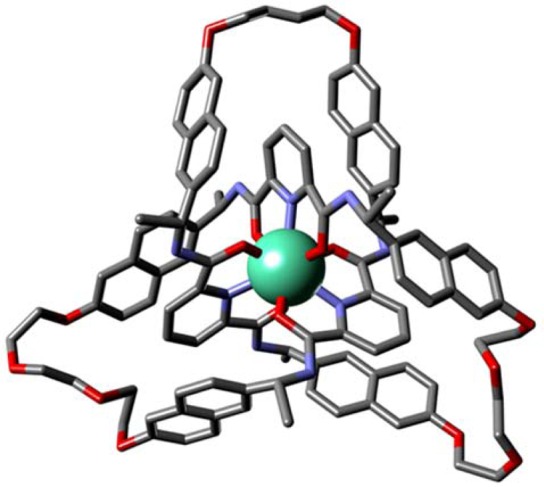
(a) X-ray crystal structure of Λ-Eu(R6)-2(CF3SO3)3 shown in the framework representation. Hydrogen atoms, solvent molecules, and counteranions have been omitted for clarity. Selected metal–donor atom bond lengths (Å): Eu–O 2.33(2) × 3, 2.39(2) × 3; Eu–N 2.52(2) × 2, 2.52(1).
Ligand 2 is a rare example of an enantiomerically pure trefoil knot.6,7 A pentafoil knot was recently employed in anion binding catalysis,16 but the chirality of molecular knots has not previously been exploited in asymmetric catalysis. As lanthanide salts have been widely used as Lewis acids to promote asymmetric Mukaiyama aldol reactions, in some cases with high enantioselectivities,17 we first investigated the efficacy of Λ-Eu(R6)-2(CF3SO3)3 as a chiral catalyst for the reaction of 4-nitrobenzaldehyde (3) with silyl enol ether 4 (Figure 3).18 Solvent choice proved crucial for the catalysis;19 5:2 methanol/acetonitrile gave both the highest conversions and the most promising levels of enantioenrichment. In comparison with both open complex Λ-Eu-6 (Figure 3 table, entry 2) and the previously reported7d trefoil knot, Λ-Eu-7 (Figure 3 table, entry 3), Λ-Eu(R6)-2 generated product 5 with improved enantiomeric enrichment (65:35 er; Figure 3 table, entry 1). In each case, enrichment was observed only in the syn diastereomer,20 with the anti diastereomer formed racemically.21 Introducing additional steric bulk into the enol ether (8) improved the enantioselectivity (83:17 er for syn-9; Figure 4). Less activated aldehydes (replacing NO2 with H or Me) gave lower yields and favored the anti adduct (10 and 11; Figure 4), although the degree of enantioselectivity in the syn diastereomer was maintained. p-Bromobenzaldehyde proved essentially unreactive under the reaction conditions employed (12; Figure 4).
Figure 3.
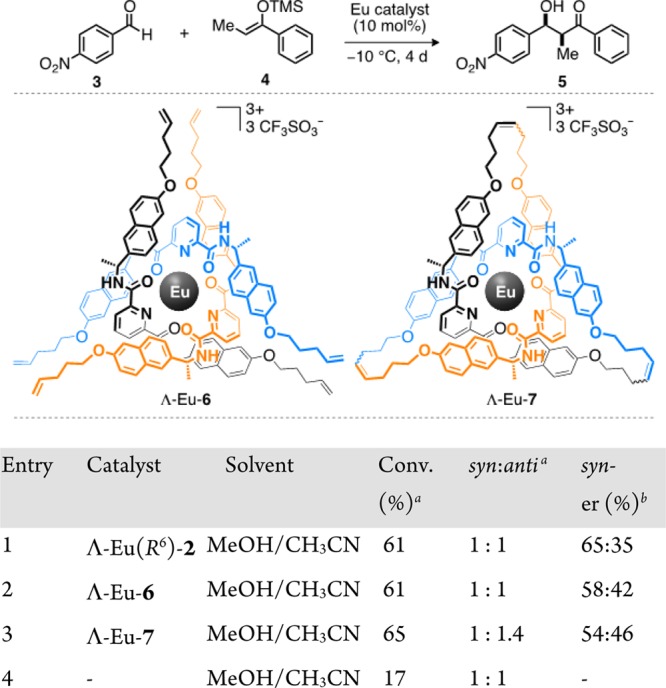
Europium-ligand-catalyzed Mukaiyama aldol reactions. Reaction conditions: 4-nitrobenzaldehyde (1.0 equiv) and trimethyl(1-phenylpropenyloxy)silane (1.0 equiv) at −10 °C for 4 days. aDetermined by 1H NMR analysis. bDetermined by chiral HPLC.
Figure 4.
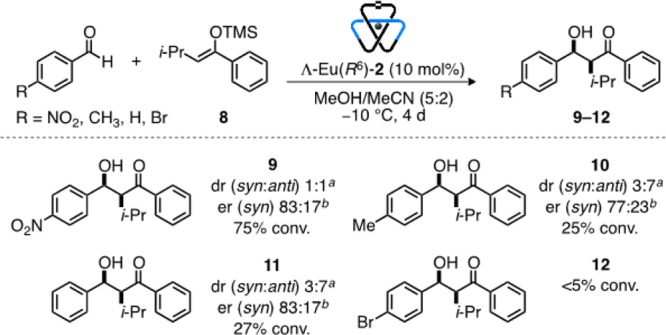
Chiral trefoil knot Λ-Eu(R6)-2(CF3SO3)3-catalyzed asymmetric Mukaiyama aldol reactions. Reaction conditions: 1.0 equiv of aldehyde and 1.5 equiv of trimethyl(3-methyl-1-phenylbutenyloxy)silane at −10 °C for 4 days. aDetermined by 1H NMR analysis. bDetermined by chiral HPLC. In all cases the relative stereochemistry of the most enantioenriched diastereomer is shown.
To probe the mechanism of enantioselective knot catalysis, we determined the accessibility of the lanthanide ion bound within the chiral pocket of the knot. Luminescence decay lifetime measurements22 in MeOH and MeOD were used to determine the number of solvent molecules bound to the lanthanide core of complexes Λ-Eu(R6)-2, Λ-Eu-6, and Λ-Eu-7 (see the Supporting Information). For the trefoil knots Λ-Eu(R6)-2 and Λ-Eu-7, q values of 0.8 and 1.1 were obtained, respectively, indicating that a solvent molecule rapidly and reversibly binds to the lanthanide ion despite the fact that it is at the center of the trefoil knot.22 As the metal ion remains accessible while bound within the chiral pocket of the knot, it may be that the Mukaiyama aldol reaction is promoted through coordination of the aldehyde to the lanthanide.23 In contrast, Λ-Eu-6 gave q values of 0.9 and 3.3, indicative of two species in slow exchange, one bound to one solvent molecule and one bound to three. Presumably the more highly solvated lanthanide ion results from transient loss of one of the 2,6-pyridinedicarboxamide groups. The continuous covalent backbone of the knotted ligand thus helps to maintain the well-defined chiral environment around the lanthanide ion, which in turn may help to maximize the enantiomeric enrichment of the syn product.
In summary, the stereochemistry of chiral centers within a ligand strand has been used to control the handedness of an overhand knot tied in the strand through complexation with a lanthanide ion. Joining the ends of the overhand knot by RCM resulted in a trefoil knot of single handedness in 90% yield. The chiral trefoil knot–lanthanide complex is an effective catalyst for the asymmetric Mukaiyama aldol reaction. The ability to tie knots of single handedness in molecular strands should facilitate the investigation of topological stereochemistry in fields where the transfer of chiral information is important (such as asymmetric catalysis, chiral recognition, chiral liquid crystal phases, and materials for nonlinear optics).
Acknowledgments
We thank the European Research Council (ERC) and the Engineering and Physical Sciences Research Council (EPSRC) for funding (EP/H021620/2), the EPSRC National Mass Spectrometry Service Centre (Swansea, U.K.) for high-resolution mass spectrometry, and Dr. Louise Natrajan and Dr. Jennifer E. Jones for their assistance with lifetime measurements.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.6b08421.
Author Contributions
† G.G.-R., S.H., M.O.K., and G.Z. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Lim N. C. H.; Jackson S. E. J. Phys.: Condens. Matter 2015, 27, 354101. 10.1088/0953-8984/27/35/354101. [DOI] [PubMed] [Google Scholar]
- Virnau P.; Mirny L. A.; Kardar M. PLoS Comput. Biol. 2006, 2, e122. 10.1371/journal.pcbi.0020122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Fenlon E. E. Eur. J. Org. Chem. 2008, 2008, 5023–5035. 10.1002/ejoc.200800578. [DOI] [Google Scholar]; b Forgan R. S.; Sauvage J.-P.; Stoddart J. F. Chem. Rev. 2011, 111, 5434–5464. 10.1021/cr200034u. [DOI] [PubMed] [Google Scholar]; c Ayme J.-F.; Beves J. E.; Campbell C. J.; Leigh D. A. Chem. Soc. Rev. 2013, 42, 1700–1712. 10.1039/C2CS35229J. [DOI] [PubMed] [Google Scholar]
- Adams C. C.The Knot Book: An Elementary Introduction to the Mathematical Theory of Knots; American Mathematical Society: Providence, RI, 2004. [Google Scholar]
- a Dietrich-Buchecker C. O.; Sauvage J.-P. Angew. Chem., Int. Ed. Engl. 1989, 28, 189–192. 10.1002/anie.198901891. [DOI] [Google Scholar]; b Dietrich-Buchecker C. O.; Guilhem J.; Pascard C.; Sauvage J.-P. Angew. Chem., Int. Ed. Engl. 1990, 29, 1154–1156. 10.1002/anie.199011541. [DOI] [Google Scholar]; c Dietrich-Buchecker C. O.; Sauvage J.-P.; Kintzinger J.-P.; Maltèse P.; Pascard C.; Guilhem J. New J. Chem. 1992, 16, 931–942. [Google Scholar]; d Ashton P. R.; Matthews O. A.; Menzer S.; Raymo F. M.; Spencer N.; Stoddart J. F.; Williams D. J. Liebigs Ann./Recl. 1997, 1997, 2485–2494. 10.1002/jlac.199719971210. [DOI] [Google Scholar]; e Rapenne G.; Dietrich-Buchecker C.; Sauvage J.-P. J. Am. Chem. Soc. 1999, 121, 994–1001. 10.1021/ja982239+. [DOI] [Google Scholar]; f Safarowsky O.; Nieger M.; Fröhlich R.; Vögtle F. Angew. Chem., Int. Ed. 2000, 39, 1616–1618. . [DOI] [PubMed] [Google Scholar]; g Adams H.; Ashworth E.; Breault G. A.; Guo J.; Hunter C. A.; Mayers P. C. Nature 2001, 411, 763. 10.1038/35081143. [DOI] [PubMed] [Google Scholar]; h Brüggemann J.; Bitter S.; Müller S.; Müller W. M.; Müller U.; Maier N. M.; Lindner W.; Vögtle F. Angew. Chem., Int. Ed. 2007, 46, 254–259. 10.1002/anie.200601938. [DOI] [PubMed] [Google Scholar]; i Barran P. E.; Cole H. L.; Goldup S. M.; Leigh D. A.; McGonigal P. R.; Symes M. D.; Wu J.; Zengerle M. Angew. Chem., Int. Ed. 2011, 50, 12280–12284. 10.1002/anie.201105012. [DOI] [PubMed] [Google Scholar]; j Prakasam T.; Lusi M.; Elhabiri M.; Platas-Iglesias C.; Olsen J.-C.; Asfari Z.; Cianférani-Sanglier S.; Debaene F.; Charbonnière L. J.; Trabolsi A. Angew. Chem., Int. Ed. 2013, 52, 9956–9960. 10.1002/anie.201302425. [DOI] [PubMed] [Google Scholar]; k Ponnuswamy N.; Cougnon F. B. L.; Pantoş G. D.; Sanders J. K. M. J. Am. Chem. Soc. 2014, 136, 8243–8251. 10.1021/ja4125884. [DOI] [PubMed] [Google Scholar]; l Bilbeisi R. A.; Prakasam T.; Lusi M.; El Khoury R.; Platas-Iglesias C.; Charbonnière L. J.; Olsen J.-C.; Elhabiri M.; Trabolsi A. Chem. Sci. 2016, 7, 2524–2531. 10.1039/C5SC04246A. [DOI] [PMC free article] [PubMed] [Google Scholar]; For other small-molecule knots, see:; m Ayme J.-F.; Beves J. E.; Leigh D. A.; McBurney R. T.; Rissanen K.; Schultz D. Nat. Chem. 2012, 4, 15–20. 10.1038/nchem.1193. [DOI] [PubMed] [Google Scholar]; n Ayme J.-F.; Beves J. E.; Leigh D. A.; McBurney R. T.; Rissanen K.; Schultz D. J. Am. Chem. Soc. 2012, 134, 9488–9497. 10.1021/ja303355v. [DOI] [PubMed] [Google Scholar]; o Engelhard D. M.; Freye S.; Grohe K.; John M.; Clever G. H. Angew. Chem., Int. Ed. 2012, 51, 4747–4750. 10.1002/anie.201200611. [DOI] [PubMed] [Google Scholar]; p Ayme J.-F.; Beves J. E.; Campbell C. J.; Leigh D. A. Angew. Chem., Int. Ed. 2014, 53, 7823–7827. 10.1002/anie.201404270. [DOI] [PMC free article] [PubMed] [Google Scholar]; q Ayme J.-F.; Gil-Ramírez G.; Leigh D. A.; Lemonnier J.-F.; Markevicius A.; Muryn C. A.; Zhang G. J. Am. Chem. Soc. 2014, 136, 13142–13145. 10.1021/ja506886p. [DOI] [PubMed] [Google Scholar]; r Ayme J.-F.; Beves J. E.; Campbell C. J.; Gil-Ramírez G.; Leigh D. A.; Stephens A. J. J. Am. Chem. Soc. 2015, 137, 9812–9815. 10.1021/jacs.5b06340. [DOI] [PubMed] [Google Scholar]
- For the separation of molecular-knot enantiomers, see:; a Rapenne G.; Dietrich-Buchecker C.; Sauvage J.-P. J. Am. Chem. Soc. 1996, 118, 10932–10933. 10.1021/ja961898o. [DOI] [Google Scholar]; b Dietrich-Buchecker C.; Rapenne G.; Sauvage J.-P.; De Cian A.; Fischer J. Chem. - Eur. J. 1999, 5, 1432–1439. . [DOI] [Google Scholar]; c Vögtle F.; Hünten A.; Vogel E.; Buschbeck S.; Safarowsky O.; Recker J.; Parham A.-H.; Knott M.; Müller W. M.; Müller U.; Okamoto Y.; Kubota T.; Lindner W.; Francotte E.; Grimme S. Angew. Chem., Int. Ed. 2001, 40, 2468–2471. . [DOI] [PubMed] [Google Scholar]
- a Perret-Aebi L.-E.; von Zelewsky A.; Dietrich-Buchecker C.; Sauvage J.-P. Angew. Chem., Int. Ed. 2004, 43, 4482–4485. 10.1002/anie.200460250. [DOI] [PubMed] [Google Scholar]; b Feigel M.; Ladberg R.; Engels S.; Herbst-Irmer R.; Fröhlich R. Angew. Chem., Int. Ed. 2006, 45, 5698–5702. 10.1002/anie.200601111. [DOI] [PubMed] [Google Scholar]; c Ponnuswamy N.; Cougnon F. B. L.; Clough J. M.; Pantoş G. D.; Sanders J. K. M. Science 2012, 338, 783–785. 10.1126/science.1227032. [DOI] [PubMed] [Google Scholar]; d Zhang G.; Gil-Ramírez G.; Markevicius A.; Browne C.; Vitorica-Yrezabal I. J.; Leigh D. A. J. Am. Chem. Soc. 2015, 137, 10437–10442. 10.1021/jacs.5b07069. [DOI] [PubMed] [Google Scholar]
- For lanthanide template synthesis of other interlocked molecular architectures, see:; a Lincheneau C.; Jean-Denis B.; Gunnlaugsson T. Chem. Commun. 2014, 50, 2857–2860. 10.1039/c3cc49640f. [DOI] [PubMed] [Google Scholar]; b Zapata F.; Blackburn O. A.; Langton M. J.; Faulkner S.; Beer P. D. Chem. Commun. 2013, 49, 8157–8159. 10.1039/c3cc45404e. [DOI] [PubMed] [Google Scholar]; c Langton M. J.; Blackburn O. A.; Lang T.; Faulkner S.; Beer P. D. Angew. Chem., Int. Ed. 2014, 53, 11463–11466. 10.1002/anie.201405131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer J. Nachr. Chem. 2016, 64, 741–746. 10.1002/nadc.20164046026. [DOI] [Google Scholar]
- For recent studies on diastereometric and asymmetric catalysis with rotaxanes, see:; a Galli M.; Lewis J. E. M.; Goldup S. M. Angew. Chem., Int. Ed. 2015, 54, 13545–13549. 10.1002/anie.201505464. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Cakmak Y.; Erbas-Cakmak S.; Leigh D. A. J. Am. Chem. Soc. 2016, 138, 1749–1751. 10.1021/jacs.6b00303. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Goldup S. Nat. Chem. 2016, 8, 404–406. 10.1038/nchem.2509. [DOI] [PubMed] [Google Scholar]
- Kotova O.; Kitchen J. A.; Lincheneau C.; Peacock R. D.; Gunnlaugsson T. Chem. - Eur. J. 2013, 19, 16181–16186. 10.1002/chem.201303660. [DOI] [PubMed] [Google Scholar]
- a Guo J.; Mayers P. C.; Breault G. A.; Hunter C. A. Nat. Chem. 2010, 2, 218–222. 10.1038/nchem.544. [DOI] [PubMed] [Google Scholar]; b Fenlon E. E. Nat. Chem. 2010, 2, 156–157. 10.1038/nchem.561. [DOI] [PubMed] [Google Scholar]
- Sauvage and co-workers found that the length of linkers between chelating units significantly affected the knot yield in their seminal trefoil knot synthesis program. See:; a Dietrich-Buchecker C. O.; Nierengarten J.-F.; Sauvage J.-P. Tetrahedron Lett. 1992, 33, 3625–3628. 10.1016/S0040-4039(00)92519-X. [DOI] [Google Scholar]; b Dietrich-Buchecker C. O.; Nierengarten J.-F.; Sauvage J.-P.; Armaroli N.; Balzani V.; De Cola L. J. Am. Chem. Soc. 1993, 115, 11237–11244. 10.1021/ja00077a024. [DOI] [Google Scholar]; c Albrecht-Gary A. M.; Dietrich-Buchecker C. O.; Guilhem J.; Meyer M.; Pascard C.; Sauvage J.-P. Recl. Trav. Chim. Pays-Bas 1993, 112, 427–428. 10.1002/recl.19931120622. [DOI] [Google Scholar]; d Dietrich-Buchecker C. O.; Sauvage J.-P.; De Cian A.; Fischer J. J. Chem. Soc., Chem. Commun. 1994, 2231–2232. 10.1039/c39940002231. [DOI] [Google Scholar]
- a Leonard J. P.; Jensen P.; McCabe T.; O’Brien J. E.; Peacock R. D.; Kruger P. E.; Gunnlaugsson T. J. Am. Chem. Soc. 2007, 129, 10986–10987. 10.1021/ja073049e. [DOI] [PubMed] [Google Scholar]; b El Aroussi B.; Zebret S.; Besnard C.; Perrottet P.; Hamacek J. J. Am. Chem. Soc. 2011, 133, 10764–10767. 10.1021/ja204474v. [DOI] [PubMed] [Google Scholar]; c Hamacek J.; Bernardinelli G.; Filinchuk Y. Eur. J. Inorg. Chem. 2008, 3419–3422. 10.1002/ejic.200800455. [DOI] [Google Scholar]; d Lincheneau C.; Destribats C.; Barry D. E.; Kitchen J. A.; Peacock R. D.; Gunnlaugsson T. Dalton Trans. 2011, 40, 12056–12059. 10.1039/c1dt11225b. [DOI] [PubMed] [Google Scholar]
- Bünzli J.-C. G.; Piguet C. Chem. Soc. Rev. 2005, 34, 1048–1077. 10.1039/b406082m. [DOI] [PubMed] [Google Scholar]
- Marcos V.; Stephens A. J.; Jaramillo-Garcia J.; Nussbaumer A. L.; Woltering S. L.; Valero A.; Lemonnier J.-F.; Vitorica-Yrezabal I. J.; Leigh D. A. Science 2016, 352, 1555–1559. 10.1126/science.aaf3673. [DOI] [PubMed] [Google Scholar]
- a Giuseppone N.; Van de Weghe P.; Mellah M.; Collin J. Tetrahedron 1998, 54, 13129–13148. 10.1016/S0040-4020(98)00791-1. [DOI] [Google Scholar]; b Hatanaka M.; Morokuma K. J. Am. Chem. Soc. 2013, 135, 13972–13979. 10.1021/ja407357c. [DOI] [PubMed] [Google Scholar]
- In the catalysis studies, Λ-Lu(R6)-2(CF3SO3)3 gave similar results as Λ-Eu(R6)-2(CF3SO3)3.
- a Kobayashi S. Pure Appl. Chem. 2007, 79, 235–245. 10.1351/pac200779020235. [DOI] [Google Scholar]; b Mei Y.; Averill D. J.; Allen M. J. J. Org. Chem. 2012, 77, 5624–5632. 10.1021/jo300800b. [DOI] [PubMed] [Google Scholar]
- The relative stereochemistry of the products was assigned through comparison of 5 and 11 to literature values. See:; a Shibata I.; Suwa T.; Sakakibara H.; Baba A. Org. Lett. 2002, 4, 301–303. 10.1021/ol0171214. [DOI] [PubMed] [Google Scholar]; b Ohtsuka Y.; Koyasu K.; Ikeno T.; Yamada T. Org. Lett. 2001, 3, 2543–2546. 10.1021/ol016204h. [DOI] [PubMed] [Google Scholar]
- For general principles in assigning syn versus anti diastereomers, see:Heng K. K.; Simpson J.; Smith R. A. J.; Robinson W. T. J. Org. Chem. 1981, 46, 2932–2934. 10.1021/jo00327a018. [DOI] [Google Scholar]
- Supkowski R. M.; Horrocks W. D. Jr Inorg. Chim. Acta 2002, 340, 44–48. 10.1016/S0020-1693(02)01022-8. [DOI] [Google Scholar]
- We cannot rule out hydrogen-bonding activation of the aldehyde from the amide group on the knot exterior as the mode of catalysis, but this mode of activation seems unlikely because the enantioselectivity improved when methanol was used as a cosolvent as opposed to solely non-hydrogen-bonding solvents.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


