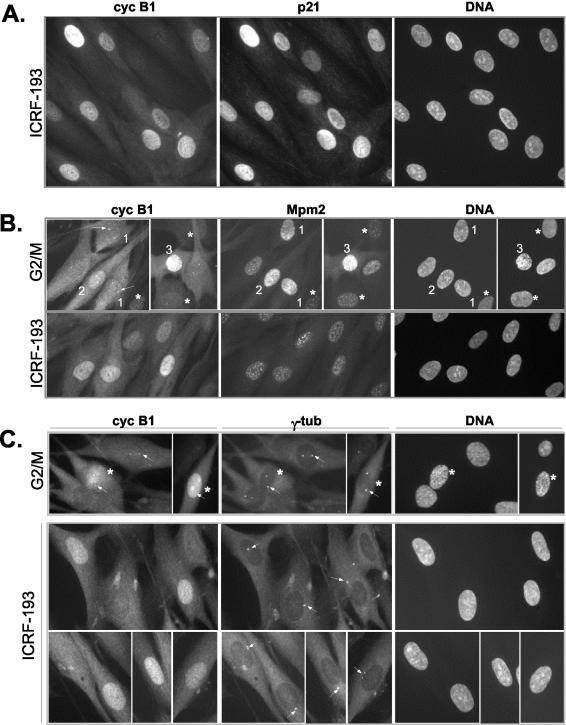
Figure 1.
Nuclear accumulation of cyclin B1 in the presence of ICRF-193, a drug that specifically provokes G2 arrest. (A) Colocalization of cyclin B1 and p21 in ICRF-193-treated synchronized NHFs. Cells were synchronized as described in MATERIALS AND METHODS, and the drug was added when the majority of the cells had passed the G1/S boundary. Control cells were fixed when most control cells had entered mitosis, whereas ICRF-193-treated cells were fixed 3 h later. Cell cycle profiles of synchronized control and drug-treated cells are shown in Supplementary Figure 1a. Cells were simultaneously stained with a mouse monoclonal anti-cyclin B1 and with a rabbit polyclonal anti-p21. (B) Indirect immunofluorescence analysis comparing subcellular localization of cyclin B1 and Mpm2 signal in control G2/M-enriched cultures and in ICRF-193-arrested NHFs. Cells were fixed in methanol and simultaneously stained with a mouse monoclonal anti-Mpm2 (Texas-Red) and with a rabbit polyclonal anti-cyclin B1 (fluorescein isothiocyanate, FITC). Cells 1-3 depict different G2/M transition stages as judged by cyclin B1 localization, the intensity of the Mpm2 signals, separation of the centrosomes (arrows), and DNA condensation levels (Hoechst). Note that only cell 3 exhibits significant DNA condensation (late prophase), whereas in the cells exhibiting increased Mpm2 staining (1 and 2) no DNA condensation is readily detectable. Arrows point at cyclin B1 localized at the centrosome. Asterisks depict cells at earlier stages of the cell cycle exhibiting a background Mpm2 signal. (C) Colocalization of cyclin B1 and γ-tubulin in control G2/M and ICRF-193-treated NHFs. Cells were fixed in methanol and simultaneously stained with a mouse monoclonal anti-γ-tubulin (Texas-Red) and with a rabbit polyclonal anti-cyclin B1 (FITC). Cells in prophase with visible DNA condensation are depicted by an asterisk (*). Note the poor separation of centrosomes (arrows) in ICRF-193-arrested cells as compared with control G2/M cells.